In a paper, ‘Understanding the Nature of Superhard Graphite,’ published in the Scientific Reports journal, an international research team headed by Artem R. Oganov from the Stony Brook University has confirmed the structure of a new type of carbon.
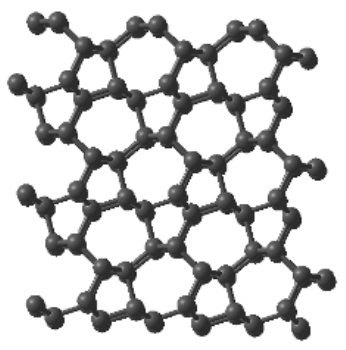 A representation of the new M-Carbon structure
A representation of the new M-Carbon structure
Using a powerful simulation method, the research team demonstrated that the properties of ‘M-carbon,’ a theoretical structure of a superhard carbon built by Oganov in 2006, was perfectly compatible with the experimental results on ‘superhard graphite,’ a transparent, colorless, superhard carbon form resulted from the compression of graphite at room temperature. This experiment was performed by Aust and Drickamer in 1963.
Oganov informed that researchers were not able to create a persuasive structural model because of ambiguous experimental data even after repeating the experiment numerous times. Oganov built M-carbon’s new low-energy superhard structure in 2006 with the help of his novel crystal structure prediction technique. This finding prompted a sequence of research works that proposed various carbon allotropes with alphabetic structures, including F-, O-, P-, R-, S-, T-, W-, X-, Y-, Z-carbons.
Properties of majority of these carbon forms matched with the experimental results on superhard graphite. Hence, more theoretical insight and better experimental data are essential to differentiate these forms, stated Oganov.
The reconstruction of graphite’s transformation into diamond is very large and is related to very high energy barrier that can be surmounted only at very high temperatures at which atoms can jump far. On the other hand, at low temperatures, graphite selects a transformation related to the lowest activation barrier. Thus, by identifying the structure that has the lowest activation barrier, it is possible to confirm the structure of the superhard graphite. For this purpose, the research team used the novel simulation technique and established that superhard graphite’s structure is identical with that of M-Carbon.
The study also provided details describing the formation mechanisms of various potential carbon allotropes. These details could prove useful to produce carbon allotropes for potential technological applications.