The latest research published in the journal ACS Sustainable Chemistry and Engineering has revealed that sustainable environmentally-friendly recycling of graphite is possible by considering the environmental footprint layout via the life cycle assessment (LCA) process.

Study: Environmental Impacts of Graphite Recycling from Spent Lithium-Ion Batteries Based on Life Cycle Assessment. Image Credit: Eaum M/Shutterstock.com
Lithium-ion batteries (LIBs) are made up of two electrode materials in which Li+ ions are reversibly intercalated back and forth, giving electrical power to an external circuit. The research indicates that LIBs' commercial success resulted in the incorporation of materials of minimal added value, such as natural graphite.
The mass of graphite accounts for approximately 15-20 percent of the weight of batteries powering electric vehicles, accounting for over 10% of the batteries' economic value.
Recycling
A priori, recycling, appears to provide apparent environmental benefits, such as increased resource efficiency, decreased carbon emissions, and waste reductions. As a result of battery recycling operations, spent graphite generally contains undesirable metal impurities (Li, Al, Co, Cu, Ni, Fe, and Mn), organic electrolytes, and polymeric binders.
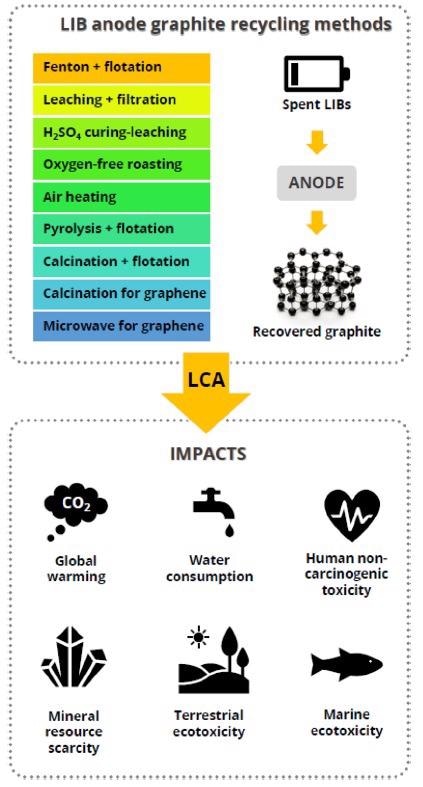
Selected nine lithium-ion battery anode recycling processes and the most representative impact categories for the conducted life cycle assessment analysis. Image Credit: Rey, I et al., Shutterstock.com
Graphite recycling/regeneration has been accomplished through a variety of techniques, such as hydrometallurgical methods based on acid-base leaching processes (for example, using acids HCl or H2SO4) or a pyrometallurgical process in which graphite is treated at temperatures above 1000 °C to gasify residual metals, metal oxides, and binders, and repair the graphite structure.
Life Cycle Assessment
The life cycle assessment (LCA) method allows for the quantification of the environmental consequences of recycling operations. LCA may be used to establish the full environmental sustainability of batteries by analyzing the contribution of recycling mechanisms to indicators like global warming, ozone layer depletion potential, ecotoxicity, eutrophication, or acidification that can be estimated.
Life Cycle Interpretation
The OpenLCA program and the Ecoinvent 3.7 Dataset were used to conduct LCA studies. The material and energy input accounting for the processing of 100 kg of graphite was determined, with 1 kg of regenerated graphite serving as a system component. This allowed for the varying graphite recovery rates for each procedure to be considered, as recovery rates ranging from 40 to 95 wt percent were achieved depending on the strategy.
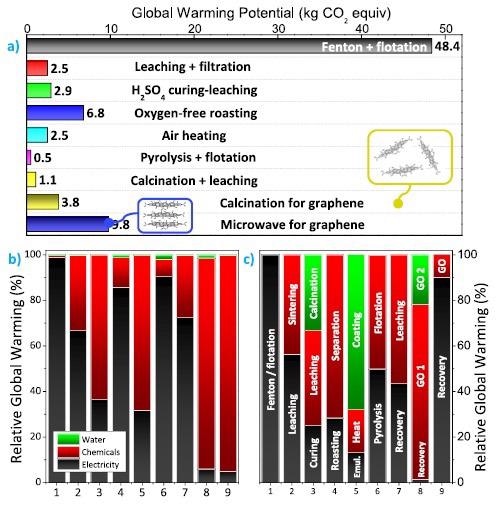
Global warming potential of graphite recycling processes from spent lithium-ion batteries: (a) GWP values in kg·CO2 equiv. emissions for 1 kg of recycled graphite from spent LIBs. For the process calcination for graphene, the impacts originating from the upcycling of spent graphite to graphene oxide are also represented by a yellow rectangle, yielding a GWP of 42.49 kg·CO2 equiv.· kggraphene‑oxide−1. For the processed microwave for graphene, the impacts account for the upcycling of spent graphite into graphite oxide (represented as a blue rectangle). (b) Relative CO2 contribution of electricity, chemicals, and water used for each graphite recycling process. (c) Relative CO2 contribution from each step during graphite recycling. Further details on each step are disclosed in the flowcharts provided in the Supporting Information as Figures S1−S9. Image Credit: Rey, I et al., Shutterstock.com
Except for the Fenton + flotation method, which gives significantly higher values, GWPs ranging from 0.53 to 9.76 kg CO2 equiv. kggraphite-1 are achieved, demonstrating unequivocally that graphite reclamation procedures are ecologically viable with pristine graphite production.
Pyrometallurgical Processes
To recycle used graphite, pyrometallurgical methods might be used. Because of the effective use of H2SO4 (only 8.9 kg), H2O2 (0.9 kg) and electricity, air heating has a low GWP of 2.56 kg. CO2 equiv.kggraphite-1 (phenolic resin ethanol solution) is used to regenerate graphite. Surprisingly, the GWP values for pyrolysis + flotation and calcination + leaching operations are as low as 0.53 and 1.08 kg.CO2 equiv.kggraphite-1, respectively.
Large volumes of inert gases, such as argon, are used in pyrometallurgical operations, leading to eutrophication, ecotoxicity, ozone depletion, human noncarcinogenic toxicity, and human carcinogenic toxicity.
When graphite is upcycled into graphene oxide, electricity is the main contributor in five of the seven techniques recovering discarded graphite (average contribution of 69%), while chemicals contribute an average of 94 percent.
Electrochemical Performance
After 50 cycles of charging and discharging at 0.1C, regenerated graphite had a capacity of 377 mA h.g-1 and retained 98.8 percent of its initial capacity. When the cycling rate was increased to 0.5C, 1C, and 2C, exceptional capacities of 320, 285, and 265 mA h.g-1 were obtained. These findings are explained by a significant specific surface area of 11.47 m2.
Although not used as energy storage devices, the upcycling of graphite into graphene-based nanocomposites is noteworthy. The calcination for graphene methods, in this context, converts graphite from spent LIBs into graphite oxide, graphene, and finally graphene oxide copper composites.
Future Perspective
Efforts are being made to prevent additional environmental damage caused by powerful acids used in hydrothermal treatments. Markey et al. employed 5 wt. percent boric acid as the leaching reagent in this framework to upcycle graphite anodes from wasted LIBs.
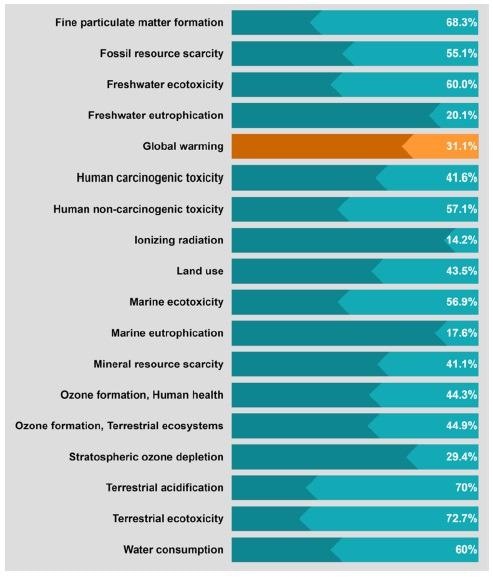
Sensitivity analysis of graphite recycling based on a modified H2SO4 curing−leaching method with a graphite:sulfuric acid ratio of 4:1 instead of the original 1:1. Image Credit: Rey, I et al., Shutterstock.com
From the study of future environmental plans and regulations, it was estimated that focusing specifically on graphite recycling, both hydrometallurgical and pyrometallurgical, requires modifications to further lower environmental damage.
At the present time, a combination of hydrometallurgy and pyrometallurgy appears to be more ecologically friendly, since it performs much better in pertinent effect categories such as global warming, freshwater toxicity, and human toxicity.
Hence, the recycling of graphite is an essential process. However, the environmental constraints are to be kept in mind. While the most ecologically friendly LIB anode technologies are chosen and scaled up, scientists and industry may be motivated by the rational design of biomass-based carbons. By combining developments in both directions, sustainable storage of energy could eventually be simplified.
References
Rey, I., Vallejo, C., Santiago, G., Iturrondobeitia, M., & Lizundia, E. (2021). Environmental Impacts of Graphite Recycling from Spent Lithium-Ion Batteries Based on Life Cycle Assessment. ACS Sustainable Chemistry and Engineering, 14488-14501. https://pubs.acs.org/doi/abs/10.1021/acssuschemeng.1c04938
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.