The California Institute of Technology (Caltech) researchers have identified the dominant working mechanism of cobalt catalysts that hold potential in the water-splitting reaction to generate hydrogen for electricity and water production.
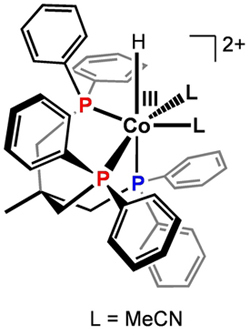 Gray's group added a set of ligands to cobalt, slowing the reaction so that they could observe a key intermediate and then determine the chemical mechanism. (credit: Caltech/Marinescu et al.)
Gray's group added a set of ligands to cobalt, slowing the reaction so that they could observe a key intermediate and then determine the chemical mechanism. (credit: Caltech/Marinescu et al.)
The study findings, appeared in the Proceedings of the National Academy of Sciences journal, pave the way to develop better water-splitting catalysts, including catalysts based on iron, a ubiquitous material. Three mechanisms have been proposed for hydrogen production utilizing the cobalt catalysts. Caltech researchers, namely Jonas Peters and Nate Lewis, suggested one mechanism, and a French team developed the second mechanism. Jillian Dempsey, a former graduate student in the group of Harry Gray, the Arnold O. Beckman Professor of Chemistry at Caltech, recently proposed the third mechanism.
So far, researchers are not able to clearly define which mechanism actually occurs and which one is dominant due to the rapid occurrence of the reactions so that they find it difficult to determine the chemical intermediates as a proof of the reactions occurring.
The cobalt catalysts are complexes comprising the metal connected to various functional groups or ligands. In the current work, Smaranda Marinescu, a Caltech postdoctoral scholar, added a new type of ligands to cobalt to reduce the speed of the reaction. This enabled the researchers to monitor the major intermediate with the help of a nuclear magnetic resonance (NMR) spectroscope.
Gray informed that the possibility to observe the key intermediate utilizing NMR and other techniques enabled the researchers to see its reaction in real time. The researchers discovered that the catalysts followed Dempsey's mechanism as the predominant pathway in the hydrogen production. In the process, a key reactive intermediate acquired an additional electron and created cobalt(II) hydride compound that became the active species of the mechanism.
Gray informed that based on the study results, researchers now have to seek designs with ligands that is capable of accepting that additional electron or those that can produce atomic cobalt with the additional electron. Gray's team is now focusing on this second method.