Jun 21 2013
Globus Medical, Inc., a leading spinal implant manufacturer, today announced the launch of LATIS™, a minimally invasive (MIS) lumbar interbody fusion spacer for patients suffering from degenerative disc disease.
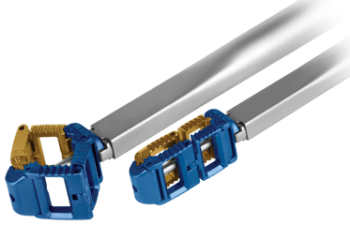 Globus Latis
Globus Latis
The implant may be inserted through an MIS transforaminal lumbar interbody fusion (TLIF) approach and expands laterally to provide a footprint and graft volume equivalent to an anterior lumbar interbody fusion (ALIF) spacer or lateral lumbar interbody fusion (LLIF) spacer.
Globus LatisThe LATIS™ spacer provides a large stable footprint and a substantial graft window, benefits typical of an ALIF or LLIF spacer, without requiring anterior access or nerve monitoring, and allows for direct decompression of nerve roots via the MIS TLIF approach. The 10mm-wide titanium implant can be inserted posteriorly and can expand in-situ up to 26mm square, offering the largest single bone graft chamber for any posterior implant on the market. Designed to reduce subsidence and migration, the spacer has a locking set screw that secures deployment at any position within the expansion range.
The LATIS™ system includes customized disc preparation and sizing instruments to simplify the procedure for an efficient MIS TLIF approach. The system includes a variety of footprints, heights, and lordotic configurations, for a customized fit for each patient. A single instrument is used for insertion, expansion, locking, and bone graft delivery.
“This new addition to our MIS portfolio is the first expandable implant on the market to offer the benefits of a traditional anterior implant, without the two-part disruptive surgical procedure. Combined with posterior stabilization using our REVOLVE® MIS pedicle screw system, the entire procedure is designed and intended to maximize preservation of the stabilizing muscles of the lower back,” commented Andrew Iott, Senior Vice President, Global Product Development.
Indications for Use:
LATIS™ Spacers are interbody fusion devices intended for use in patients with degenerative disc disease (DDD) at one or two contiguous levels of the lumbosacral spine (L2-S1). DDD is defined as discogenic back pain with degeneration of the disc confirmed by history and radiographic studies. These patients should be skeletally mature and have had at least six (6) months of non-operative treatment. In addition, these patients may have up to Grade 1 spondylolisthesis or retrolisthesis at the involved level(s). LATIS™ Spacers are to be filled with autogenous bone graft material. These devices are intended to be used with supplemental fixation.