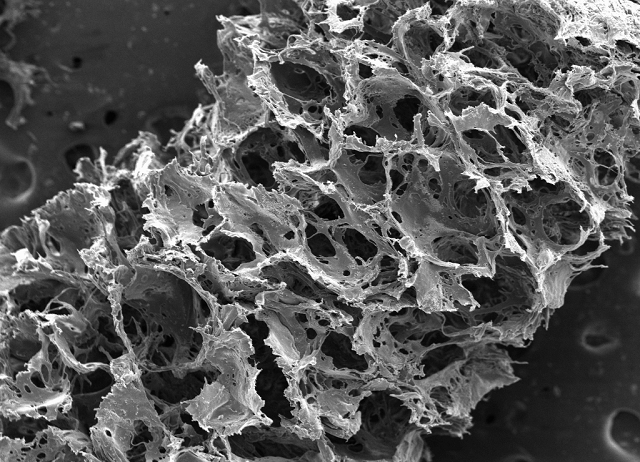
Image: Up close scaffold.
Silk-based scaffold taken with a scanning electron microscope reveals its porous, sponge-like composition. Image courtesy of Tufts University.
A group of bioengineers has successfully developed a 3D brain-like tissue that exhibits similar structural and functional properties as brain tissue in rats. The study was funded by NIH’s National Institute of Biomedical Imaging and Bioengineering (NIBIB) to set up novel biomaterials and tissue engineering models.
Developed at Tufts University’s Tissue Engineering Resource Center, this brain-like tissue can survive in the lab for over two months. To demonstrate the potential of this tissue model, the researchers used it to inspect the electrical and chemical changes that occur after a traumatic brain injury.
With the system we have, you can essentially track the tissue response to traumatic brain injury in real time. Most importantly, you can also start to track repair and what happens over longer periods of time.
Dr. David Kaplan - Stern Family Professor of Engineering - Tufts University
This innovative model can be used for studying not only normal brain function, but can also be adapted for studying the related injuries and diseases. The model could even help in the development of novel treatments for brain dysfunction.
The director of the center at Tufts University and leader of the research efforts, Dr. David Kaplan, said that this new method of studying the brain can eliminate the issues with delays in animal studies whilst the brain is extracted and prepared for research.
To develop the 3D brain-like tissue, the researchers produced a composite structure that contains two biomaterials - a collagen-based gel and a spongy scaffold made from silk protein.
The scaffold was used as a structure on which neurons could attach on their own, while the gel promotes axons to grow through it.
In order to achieve the compartmentalization of grey and white matter, the spongy scaffold was cut into a donut shape and filled with rat neurons.
The center of the donut was then filled with the collagen-based gel, which subsequently permeated the scaffold. Within a matter of days, the neurons established functional networks around the scaffold pores and transmitted longer axon projections via the center gel to join with neurons on the other side of the donut.
This resulted in a clear, well-defined white matter region that formed in the middle of the donut, which was independent from the adjacent grey matter.
After a number of weeks, experiments were performed to ascertain the function of the neurons that were growing in their 3D brain-like tissue. These neurons were then compared with the neurons grown in a 2D dish or a collagen gel-only environment.
It was found that the neurons in the 3D brain-like tissue displayed greater expression of genes involved in growth and function of neurons.
These neurons also exhibited stable metabolic activity for a period of five weeks. On the other hand, the health of the neurons grown in the gel-only setting started to deteriorate within a period of 24h.
Since the 3D brain-like tissue shared similar physical properties like the rat brain tissue, the researchers attempted to determine whether this model could be used to investigate traumatic brain injuries.
Dr. Rosemarie Hunziker, program director of Tissue Engineering at NIBIB described this development as an "exceptional feat". She went on to say that the discovery has combined a strong understanding of brain physiology with an ever increasing set of bioengineering tools which has enabled the creation of an environment which is perfect for mimiking brain function.
The study was published in the journal Proceedings of the National Academy of Sciences.