UC Davis researchers have succeeded in trapping atoms in borosilicate glass in their transition stage from a flat triangular configuration to a tetrahedron configuration when subjected to high pressure.
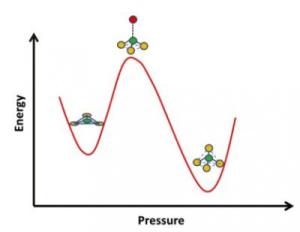 Glass has many applications that call for different properties, such as resistance to thermal shock or to chemically harsh environments. Glassmakers commonly use additives such as boron oxide to tweak these properties by changing the atomic structure of glass. Now researchers at UC Davis have for the first time captured atoms in borosilicate glass flipping from a flat triangular configuration with three oxygen atoms around one boron to a tetrahedron, via a pyramidal intermediate. Credit: Sabyasachi Sen, UC Davis
Glass has many applications that call for different properties, such as resistance to thermal shock or to chemically harsh environments. Glassmakers commonly use additives such as boron oxide to tweak these properties by changing the atomic structure of glass. Now researchers at UC Davis have for the first time captured atoms in borosilicate glass flipping from a flat triangular configuration with three oxygen atoms around one boron to a tetrahedron, via a pyramidal intermediate. Credit: Sabyasachi Sen, UC Davis
Glass is used in a wide range of applications and in order to suit a specific application, glass manufacturers fine tune its properties.
The materials science professor at UC Davis, Sabyasachi Sen, stated that the research findings help in understanding how amorphous materials such as glass react to stress at the atomic scale.
Boron oxide is used as an additive in glass manufacture to fine-tune its properties such as optical transparency, flow resistance, thermal expansion and chemical durability.
The atomic structure around boron atoms changes with pressure and temperature, flipping from a triangular structure of one boron atom enclosed by three oxygen atoms to a tetrahedral structure of one boron atom enclosed by four oxygen atoms. Until now, these structures could be analysed in either of the states but not while in transition.
A probe was designed by Sen and a graduate student, Trenton Edwards, with which NMR measurements could be made of the surrounding area of the boron atoms in glass at pressures of up to 2.5 GPa.
It was observed that when subjected to high pressure, the triangular structure deforms to a pyramid with the boron atom in a pushed-up state. That draws it nearer to an adjacent oxygen atom and the configuration is changed to a tetrahedral structure with one boron atom enclosed by four oxygen atoms.
It is surprising that even though glass is isotropic in nature and the stress applied to it is isotropic, the boron atoms move in a specific direction with regards to the remaining structure. The study was published in the journal Science.