Researchers at the Pacific Northwest National Laboratory (PNNL) have unexpectedly discovered a new semiconducting material that can improve the efficiency of fuel cells by operating at a significantly lower temperature.
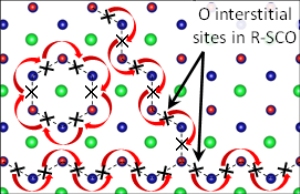 Routing: Oxygen can zigzag or take a circular route (red arrows) through this semiconducting crystal made of strontium (green), chromium (blue), and oxygen (red). Image Courtesy of Nature Communications
Routing: Oxygen can zigzag or take a circular route (red arrows) through this semiconducting crystal made of strontium (green), chromium (blue), and oxygen (red). Image Courtesy of Nature Communications
Current materials in solid oxide fuel cells require a high temperature of 800°C and must allow diffusion of oxygen.
The PNNL researchers have been trying to make a metal oxide that possessed the properties of metals. They created strontium-chromium oxide that demonstrated the properties of a semiconductor. This material allows easy diffusion of oxygen at a temperature that may help develop better fuel cells.
The researchers tried to produce strontium-chromium oxide in perovskite form – a crystalline form that has helpful electronic properties. In this form, the atoms of strontium, chromium and oxygen stack collectively in a cube, and the strontium and chromium atoms bond to the oxygen atoms.
However, the strontium chromium oxide formed into a rhombus-shaped crystal without many oxygen atoms. These oxygen vacancies formed well-defined planes that allowed outside oxygen to diffuse through at a low 250°C temperature. Oxygen vacancies at high concentrations formed a mesoscale crystalline structure that efficiently transmitted oxygen.
The researchers had been trying to produce metallic SrCrO3, but they ended up creating semiconducting SrCrO2.8. Ultra-pure crystalline films of this material were created and then analyzed using an oxygen-assisted molecular beam epitaxy deposition system at Department of Energy's Environmental Molecular Sciences Laboratory.
The scientists intend to use the knowledge gained for studying other materials like epitaxial strontium-doped lanthanum chromite, a promising material for visible light harvesting. They also plan to nanofabricate novel heterogeneous catalytic structures. The researchers have reported this study in Nature Communications.