New York University scientists have discovered that the low-temperature microscopic particles tend to melt with the rise in temperature to moderate levels and again recombine under high temperature. This invention paves the way for the development of smart materials capable of adapting to any new environment, and for providing detailed study of 3D printing technology.
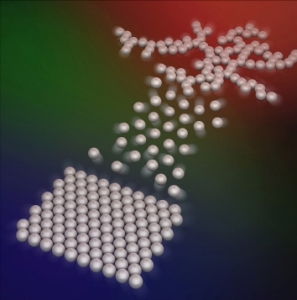 Microscopic particles that bind under low temperatures (blue: bottom left) will melt as temperatures rise to moderate levels (green: center), but re-connect under hotter conditions (red: top right), a team of NYU scientists has found. Their discovery points to new ways to create “smart materials,” cutting-edge materials that adapt to their environment by taking new forms, and to sharpen the detail of 3D printing. Image courtesy of Lang Feng.
Microscopic particles that bind under low temperatures (blue: bottom left) will melt as temperatures rise to moderate levels (green: center), but re-connect under hotter conditions (red: top right), a team of NYU scientists has found. Their discovery points to new ways to create “smart materials,” cutting-edge materials that adapt to their environment by taking new forms, and to sharpen the detail of 3D printing. Image courtesy of Lang Feng.
These findings prove the ability to manipulate even the small particles using different methods, revealing that the well-known Goldilocks Principle may not be applicable for the smallest of particles.
The research involves studying the properties of colloids and polymers – particles that are as small as one-millionth and one-billionth of a meter, respectively. These materials are of significant interest to the scientists owing to their applications in a wide range of consumer products such as colloidal dispersions in porcelain, glass, gelatin, milk and paint and for advanced engineering products such as steering light in photonics. Scientists can enhance the material properties or develop enhanced materials by studying the process colloidal or polymer formation.
The researchers investigated the colloidal crystals and polymers at temperatures between room temperature and 85°C. The polymers remain as gas hitting the larger particles at room temperature. If the distance between the particles is too small, a pressure is applied to force them to bind together.
Upon heating, the crystals melted and re-solidified on heating. The re-solidified solid appeared to be a soft, pliable Jello-like substance with the polymers binding to the colloids. The structure of the solid seemed to be more open than the crystal.
These findings show the potential to engineer the properties of materials using not only temperature, but also by employing a range of methods to manipulate the smallest of particles.
Lang Feng, the study’s lead author and an NYU doctoral student
The researchers observed enthalpic attraction in the crystals, which is nothing but the adhesive energy generating bonding between the particles at high temperatures. However, the conditions were fairly high at the mid-level temperatures to create entropic force, and too cool to initiate enthalpic attraction.
This technology has the potential to create 3D structures from two-dimensional layers. However, by improving the manipulation of particles at the microscopic level, it is possible to develop more realistic and detailed objects.
This work is published in the Nature Materials journal and supported by the National Science Foundation’s MRSEC Program.
References