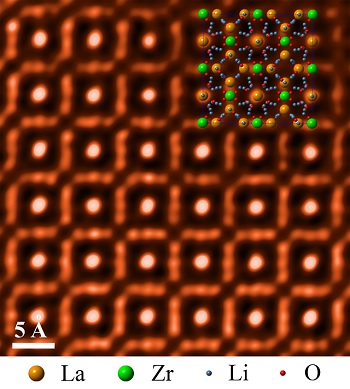
Researchers at the Oak Ridge National Laboratory have discovered that a cubic garnet material (LLZO) could be used as a critical separator material in order to develop high-energy batteries.
Many novel batteries comprise of a lithium anode and an aqueous electrolyte. However, when they are integrated into a single battery, a violent reaction occurs when the water and lithium come into contact with each other.
As a result, the lithium anode requires a protective layer. Solid electrolyte separators have previously been used to shield the lithium. However, even the widely used LISICON or LAPT separators fail under standard operating conditions.
Researchers have been trying to find an appropriate solid electrolyte separator material. A suitable material must demonstrate wide pH range stability and lithium anode compatibility. As lithium batteries have an alkaline environment, a wide pH range is required.
The research team at the Oak Ridge National Laboratory analysed the LLZO cubic garnet material using scanning transmission electron microscopy.
They immersed samples in different aqueous solutions and observed the structural changes which occurred in the LLZO material.
Over a period of time it was found that the material retained its structural stability in neutral and highly alkaline environments.
This solid electrolyte separator remains stable even for a pH value higher than 14. It gives battery designers more options for the selection of aqueous solutions and the catholyte.
Cheng Ma, ORNL Postdoctoral Associate
Previously, researchers have attempted to use diluted aqueous solutions in order to prevent the degradation of the separators but this made the battery bulky and heavy.
The LLZO cubic garnet separator does not require dilution of the aqueous electrolyte and hence increases the energy density of the battery, without making it bulky.
Batteries with high energy are required in electric grid energy storage and in electrified transportation applications.
The newly developed separator study is to be evaluated in an operating battery by the research team. This study has been published in Angewandte Chemie.