An international team of scientists have discovered a safe sodium-based material which could be used as a cost-effective alternative to the lithium-based conductors currently used in rechargeable batteries.
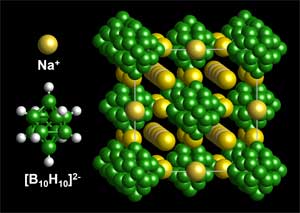 Image Credit: Udovic/NIST
Image Credit: Udovic/NIST
The new material comprises of sodium, boron and hydrogen elements which can all be easily obtained. Researchers from the National Institute of Standards and Technology (NIST), the Institute of Metal Physics, the Tohoku University, Sandia National Laboratories and the University of Maryland have collaborated on this study.
The new material, which has the chemical formula Na2B10H10, is a stable organic solid and carries a smaller risk of leakage or explosion when compared to traditional batteries. In addition, it provides an increased power output than other sodium-based solids as when heated, it conducts sodium ions very effectively.
The atoms in the sodium-based hydride are tightly packed at room temperature but they repack when they are heated to a temperature near the boiling point of water. In this process, a great number of large connected corridors are created through which charged sodium ions can easily travel.
In batteries, charged ions carry electrical current which enables the new novel sodium-based hydride material to perform over 20 times better than other existing sodium-based complex hydrides. It is comparable in performance to efficient solid lithium-based hydride batteries.
Currently, this battery can only operate above its phase transition temperature but researchers are trying to reduce this operational transition temperature to room temperature. Further research will also try to optimise the performance of the battery by fine-tuning the properties of the sodium-based complex hydride material.
This study has been published in the journal Advanced Materials.