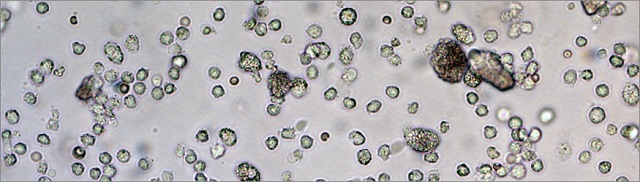
 A microscope image shows many of the immune system's dendritic cells that were collected from a 3D scaffold three days after in vivo injection. The 3D scaffold effectively recruits and activates the dendritic cells to trigger an immune response against specific cells, such as cancerous cells.
Credit: Wyss Institute at Harvard University
A microscope image shows many of the immune system's dendritic cells that were collected from a 3D scaffold three days after in vivo injection. The 3D scaffold effectively recruits and activates the dendritic cells to trigger an immune response against specific cells, such as cancerous cells.
Credit: Wyss Institute at Harvard University
An injectable, programmable biomaterial that could fight and help prevent cancer, HIV and other infectious diseases has been developed by researchers belonging to Harvard University’s Wyss Institute for Biologically Inspired Engineering and Harvard's School of Engineering and Applied Sciences. The programmable biomaterial that can be delivered through non–surgical injection, has the ability to assemble spontaneously into a 3D structure in vivo.
Cancer has the ability to evade attacks by the body's immune system. This enables tumors to increase and spread, and makes the cancer deadly. Immunotherapy involves inducing the immune system to fight cancer by making it go into an attack mode and also build immune resistance to cancer cells.
We can create 3D structures using minimally–invasive delivery to enrich and activate a host's immune cells to target and attack harmful cells in vivo.
David Mooney, Ph.D., Wyss Institute Core Faculty member
Chemical and biological drugs can be loaded on to mesoporous silica rods (MSRs), which are biodegradable, tiny rod–like structures. These can be delivered beneath the skin using a needle. At the place where the vaccination occurs, these rods assemble and form a 3D scaffold. This is similar to pouring of matchsticks into a pile. The stack of MSRs has porous spaces that are sufficiently large enough to get filled with dendritic cells. When the body detects any harmful presence these "surveillance" cells trigger an immune response.
Nano–sized mesoporous silica particles have already been established as useful for manipulating individual cells from the inside, but this is the first time that larger particles, in the micron–sized range, are used to create a 3D in vivo scaffold that can recruit and attract tens of millions of immune cells.
Jaeyun Kim, Ph.D., an Assistant Professor of Chemical Engineering at Sungkyunkwan University
When MSRs are synthesized in the lab, they are built with nanopores. Any variety of drugs including, specific cytokines, large protein antigens, or oligonucleotides can be used to fill these nanopores. This method allows numerous potential combinations for treating different types of infections.
The co-lead author of the study, Aileen Li, who is a graduate student pursuing her Ph.D. in bioengineering at Harvard SEAS, states that "Although right now we are focusing on developing a cancer vaccine, in the future we could be able to manipulate which type of dendritic cells or other types of immune cells are recruited to the 3D scaffold by using different kinds of cytokines released from the MSRs. By tuning the surface properties and pore size of the MSRs, and therefore controlling the introduction and release of various proteins and drugs, we can manipulate the immune system to treat multiple diseases."
After the dendritic cells are recruited from the body by the 3D scaffold, the release of drugs in the MSRs takes place. Their "surveillance" gets tripped and this induces an immune response. The dendritic cells move to the lymph nodes and raise an alarm, which induces the immune system to attack cancerous cells or other specifically targeted cells. The MSRs biodegrade naturally and would dissolve within a couple of months.
During tests, the 3D vaccine has been found to be very effective in mice. The injectable 3D scaffold had the capability to recruit and attract dendritic cells in millions. These were then dispersed to the lymph nodes to trigger a huge immune response.
When faced with an emerging infectious disease problem, these vaccines could be quickly and easily manufactured. "We anticipate 3D vaccines could be broadly useful for many settings, and their injectable nature would also make them easy to administer both inside and outside a clinic," said Mooney.
This method could also be used as a preventative measure for building the immune resistance of the body before infection takes place.
Injectable immunotherapies that use programmable biomaterials as a powerful vehicle to deliver targeted treatment and preventative care could help fight a whole range of deadly infections, including common worldwide killers like HIV and Ebola, as well as cancer. These injectable 3D vaccines offer a minimally invasive and scalable way to deliver therapies that work by mimicking the body’s own powerful immune–response in diseases that have previously been able to skirt immune detection.
Wyss Institute Founding Director Donald Ingber, M.D., Ph.D.
Sarah A. Lewin of the Wyss Institute, Youngjin Choi of Sungkyunkwan University in Korea, Glenn Dranoff of the Dana-Farber Cancer Institute and Harvard Medical School and Catia S. Verbeke of Harvard SEAS and the Wyss Institute were the additional co-authors of the study.
The National Institutes of Health, the National Science Foundation, the National Research Foundation of Korea and the Wyss Institute for Biologically Inspired Engineering at Harvard University have supported this study.
The researchers have published this study in Nature Biotechnology.
This Story Around the Web
References