Researchers from the University of Maryland, Baltimore County (UMBC) have harnessed the power of natural materials to build better lithium ion batteries.
The team has isolated a peptide that has the capability to bind strongly to nanosized particles of lithium manganese nickel oxide (LMNO). This material can be used to manufacture the cathode component in high performing batteries.
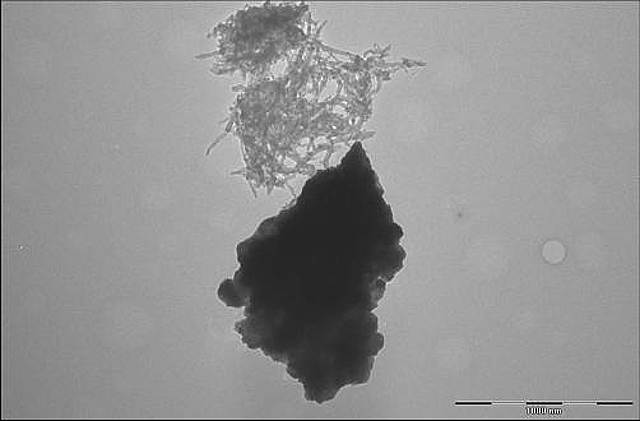 Lithium manganese nickel oxide and carbon nanotubes clump separately, with no specific interactions. Credit: Evgenia Barannikova/UMBC
Lithium manganese nickel oxide and carbon nanotubes clump separately, with no specific interactions. Credit: Evgenia Barannikova/UMBC
This peptide, which is a type of biological molecule, binds onto nanosized LMNO particles and then connects these particles to a battery electrode’s conductive components. This enhances the potential stability and power of the electrode.
Biology provides several tools for us to solve important problems. By mimicking biological processes we can find better solutions.
Evgenia Barannikova, graduate student at UMBC
Nanoscale materials can often be difficult to work with due to their tiny scale - it is not straightforward to manipulate them or even hold them in place.
However, when compared to bulk material electrodes, nanostructured electrodes in Li-ion batteries demonstrate numerous advantages. They have a large surface area that enables more active sites for electrochemical reactions to take place, and the charge-carrying particles need to travel only shorter distances. These properties lead to batteries that have a longer life and that are comparatively lighter.
Barannikova and her colleagues used peptides to address the challenge of nanoscale manufacturing. Peptides occur naturally, and are made of a chain of smaller amino acid molecules.
Depending on their amino acid sequence, peptides are able to bind themselves to various types of organic and inorganic materials. In the human body, these peptides play a number of roles like regulating blood sugar and signaling in the brain. Insulin and other hormones are composed of peptides.
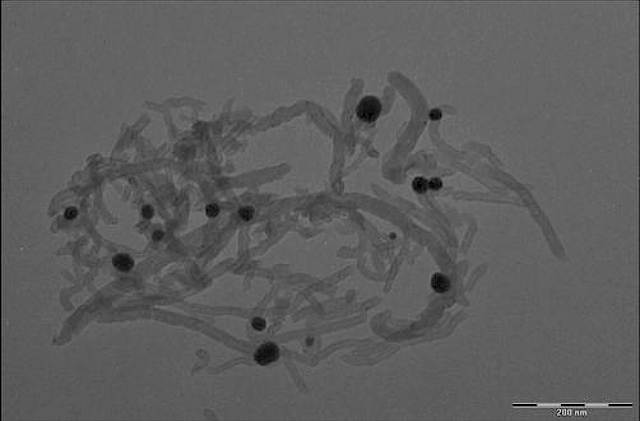
Multifunctional binding peptide binds the dispersed carbon nanotubes to lithium manganese nickel oxide particles. Credit: Evgenia Barannikova/UMBC
Barannikova states that the manner in which mollusks and other such organisms use peptides for controlling their shell growth was one among the other factors that inspired her research. Mollusks demonstrate amazing control when building complex nano- and macrostructures from calcium carbonate and other such inorganic materials.
The research team followed mollusks’ approach, however in order to find out a suitable peptide, they had to utilize some lab-bench wizardry. This is because snails don’t use lithium manganese nickel oxide to make their shells.
In order to find a peptide that would latch on to lithium manganese nickel oxide strongly, the researchers utilized the "Phage Display" procedure to screen over a billion possible peptides. A laboratory supply company had commercially produced a "peptide library" from which the researchers selected their peptide. This "peptide library" contains a large number of amino acid sequences combined randomly and incorporated into a protein created by the M13 bacteriophage virus.
The research team combined the "peptide library" and a metal oxide sample in order to isolate a peptide that had the capability to bind to lithium manganese nickel oxide. The peptides that did not stick to the lithium manganese nickel oxide were washed away. The newly-discovered peptide was then combined with a peptide that had been previously isolated, and could bind to carbon nanotubes. In Li-ion electrodes the carbon nanotubes could function as conductive nanowires.
The peptide formed from this process could form a bridge, which could bind to the carbon nanotubes and the lithium manganese nickel oxide nanoparticles, and also keep them near each other so that a connection could be maintained through multiple charging cycles. The researchers consider that maintenance of highly organized nanoscale architecture will help enhance the lifetime, cycling stability and power of future Li-ion batteries, while allowing them to be smaller in size.
Currently, the researchers are testing the performance of the new cathodes. Barannikova intends to create an anode using similar techniques and then integrate the two components. "I hope to demonstrate an entire biotemplated battery in my Ph.D. thesis," she said.
The researchers have presented the findings of their study as a poster titled "Solid-binding Peptides as a Biotemplate for Li-ion Battery Electrodes" at the 59th annual meeting of the Biophysical Society in Baltimore, Maryland.