The University of Houston researchers have developed a versatile electron-transporting polymer that is highly conductive. This has been a mystery to researchers for a very long time and this discovery holds promise in ultrafast battery applications.
 Rational combination of advantages of state-of-the-art polymers has resulted in highly electronically conducting polymers that could enable a battery to be 80 percent charged within 6 seconds, and fully charged in another 18 seconds
Rational combination of advantages of state-of-the-art polymers has resulted in highly electronically conducting polymers that could enable a battery to be 80 percent charged within 6 seconds, and fully charged in another 18 seconds
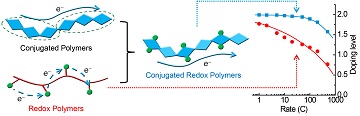
This innovation is based on a “conjugated redox polymer” design using a naphthalene biothiophene polymer. This polymer was previously used for applications such as solar cells and transistors.
The researchers used lithium ions as dopant and observed that the polymers provide excellent electronic conductivity, reversibility and stability across several thousand cycles of charging and discharging.
The research was published in the Journal of the American Chemical Society and chosen as ACS Editors’ Choice for open access. Yan Yao, lead author of the paper and assistant professor of electrical and computer engineering at the UH Cullen College of Engineering said that the research acknowledges the challenges of electron-transport conducting polymers.
Even after realizing the capabilities of functional organic polymers, researchers were unable to develop an efficient electron-transport conducting polymer that can be paired with the hole-transporting polymers. However, the lithium-doped naphthalene-bithiophene polymer was found to have high stability across 3000 cycles of discharging and charging energy in addition to good electronic conductivity.
The invention could find potential applications as a cost-effective alternative to conventional inorganic-based energy devices such as lithium batteries. Yao said that this can ultimately lead to the development of inexpensive consumer devices and electric cars.
The research team of Yao who is also the principal investigator for the Texas Center for Superconductivity at UH is involved in producing sustainable/green organic materials for efficient production and storage of energy.
Yanliang Liang, a research associate at UH and first author on the paper, said that the aim of the research is not to directly challenge traditional lithium-ion batteries. “We are trying to demonstrate a new direction,” he said.
According to Liang, the choice of materials including silicon and cobalt-based compounds used in conventional inorganic metal-based batteries adds to the production cost of the energy storage devices.
By contrast, organic polymers can be processed at low temperatures which considerably lower the cost. They also ensure reduced CO2 emissions. Unlike conventional materials that are finite in nature, organic polymers can be produced from biomass.
Organic -conjugated polymers are emerging as a materials class for energy-related applications, enabling a path to a more sustainable energy landscape without the need of energy-intensive, expensive and sometimes toxic metal-based compounds, concluding that a model polymer, P(NDI2OD-T2), was stably and reversibly n-doped to a high doping level of 2.0, a significant progress for electron-transporting π-conjugated polymers. … With rational molecular design, π-conjugated redox polymers will establish new design space in polymer chemistry and see wide-spread applications, especially in energy-related ones such as batteries, supercapacitors and thermoelectrics.
The polymer used in the research was supplied by the researchers from an Illinois-based technology company, Polyera Corporation. The polymer was invented in 2009. Besides naphthalene-bithiophene's applications in transistors and other devices from the time of its discovery, this is the first time it has been converted for energy storage purposes. The conversion was carried out by adding lithium and increasing the doping level of polymer from 0.1 to 2.0.
Excellent results were reported from the experiment. Under practical measurement conditions, the polymer was proved to show the most rapid charge-discharge performance for any organic material. This performance enables full-charging of the battery in 24s and 80% charging in 6s.
Yao said that the conventional inorganic batteries however have an ability to store more energy when compared to the organic battery. The work will further involve enhancing the storage capacity of the material. The team will also continue to work on basic scientific research to gain in-depth knowledge about the polymer.
References