Economical fabrication of electronics on curved or flexible surfaces could become a reality due to printed electronics.
This will allow for the use of electronics in a wide range of applications, which could even include fabricating smartwatches or mobile phones using a printer at home. However, in order for these advancements to become reality, the cost and quality of available conductive inks will need to be improved.
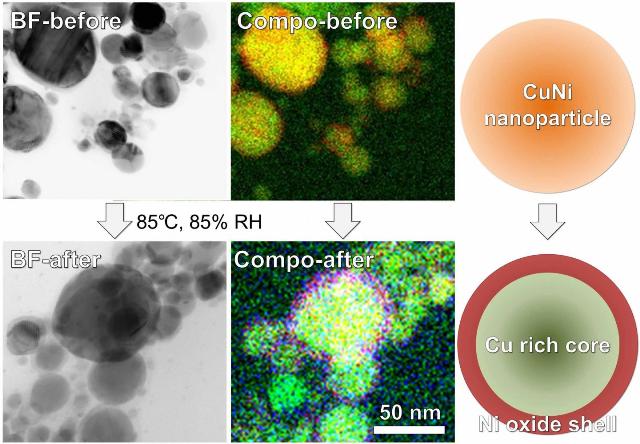 These are bright-field (BF) scanning transmission electron microscope images, composed (Compo) elemental mappings, and illustrations of Cu alloy nanoparticles containing 30 percent Ni before and after oxidation treatment at 85°C and 85 percent relative humidity. (CREDIT COPYRIGHT (C) 2016 TOYOHASHI UNIVERSITY OF TECHNOLOGY. ALL RIGHTS RESERVED.)
These are bright-field (BF) scanning transmission electron microscope images, composed (Compo) elemental mappings, and illustrations of Cu alloy nanoparticles containing 30 percent Ni before and after oxidation treatment at 85°C and 85 percent relative humidity. (CREDIT COPYRIGHT (C) 2016 TOYOHASHI UNIVERSITY OF TECHNOLOGY. ALL RIGHTS RESERVED.)
A team of researchers from Toyohashi University of Technology and Duke University have developed a method to manufacture unique copper alloy nanoparticles. These can be used as the key constituent of cost-effective, oxidation-resistant conductive inks.
The collaborative team produced the nanoparticles by electrically exploding
twisted or alloy metal wires in water containing Vitamin C, which is a mild reducing agent. Then, the decrease in conductivity was measured under high humidity and high temperature.
"We have been working on developing a 'wire explosion' method to produce novel metal nanoparticles. Then, we found that some of the produced copper alloy nanoparticles possessed both high oxidation resistance and low electrical resistance," explains Assistant Professor Go Kawamura. "Moreover, the nanoparticles have the advantage of being inexpensive because the production process is very economical and environmentally friendly."
Consequently, the electrical conductivity of copper nanoparticles alloyed with 5% Ag, 1% Sn, 5% Ni, or 30% Ni matched that of copper. However, the nanoparticles were able to retain their conductivity after 24 hours at 85% relative humidity and 85 °C, unlike copper.
Improving the oxidation resistance and electrical conductivity could lead the copper alloy nanoparticles fabricated by wire explosion to be used in the production of high-performance, economical conductive inks, which will help to progress the printed electronics industry.
The team hopes that its research findings will inspire further study of using the technique of integrating wire explosion with chemical modification of the explosion medium to control the surface chemistry and composition of nanoparticles.