Jan 29 2016
Oil and water don't mix, but when one is finely dispersed in the other a liquid mixture is produced with useful properties. An emulsion comprising of tiny droplets of one of the liquids immersed in the other is the usual form, found in salad dressings, cosmetics and industrial lubricants.
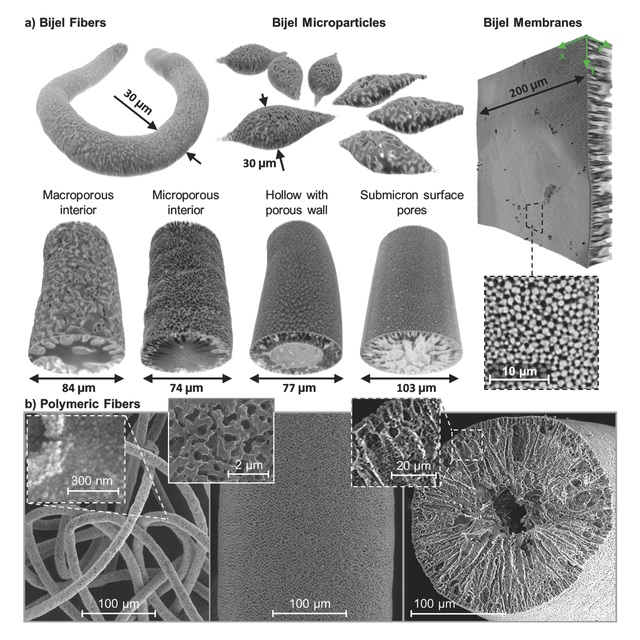 STRIPS makes it easier to control the overall shape of the bijel, enabling it to take the form of blobs, membranes or fibers
STRIPS makes it easier to control the overall shape of the bijel, enabling it to take the form of blobs, membranes or fibers
Complex emulsions can also be made; controlling the shape of the interfaces between the two liquids unlocks new behaviors and applications. Thanks to research from the University of Pennsylvania, School of Engineering and Applied Science, a special type of emulsion is becoming easier to make.
Referred to as a bicontinuous interfacially jammed emulsion gel - or bijel - the new emulsion is a type of liquid conveyor belt for continuous chemical reactions. Rather than isolated droplets, both the oil and water phases in these materials consist of densely intertwined, but fully connected networks that other molecules can flow through. A particle layer “jammed” at the interface of the oil and water networks can prevent their further dispersion, and can also act as a catalyst for such reactions.
Only recently has this complex structure been made possible, aided by advances in soft matter physics, and until now it was restricted to a small set of compatible oils. The Penn team’s method for creating bijels, using ethanol rather than precise temperature changes to drive the networks formation, works with a wide array of oils, allowing it to be used for new applications.
The method makes it easier to control the overall shape of the bijel, allowing it to take the form of membranes, blobs or fibers. The method also enables control of the internal network dimensions, potentially fine-tuning it to a particular application.
The Penn team includes Daeyeon Lee, an associate professor in Penn Engineering’s Department of Chemical and Biomolecular Engineering; Kathleen Stebe, the Richer & Elizabeth Goodwin Professor of Chemical and Biomolecular Engineering; and Martin Haase, a postdoctoral researcher who works in both of their labs. Their results were published in Advanced Materials.
Originally a team of researchers at the University of Edinburgh formulated bijels, made using a process called as thermally induced spinodal decomposition. In this method, heat is used to force the water and oil to mix, and then separated by lowering the mixture temperature. Injecting particles, which travel to intertwined interfaces between the two liquids, stops the process before the separation of oil and water into discrete droplets.
Solvent Transfer Induced Phase Separation
The problem with this process is that it's very delicate. While there are a handful of special sets of oil and water that will do the temperature trick, there are hundreds of sets that work with our method. We went from a finicky, delectate space to a workhorse space that we can use again and again.
Kathleen Stebe, Richer & Elizabeth Goodwin Professor of Chemical and Biomolecular Engineering, Penn University
The team brainstormed potential research projects that involved these elements, and the manufacturing of bijel jumped out at them. The method they created, called the solvent transfer-induced phase separation (STRIPS), uses ethanol instead of heat for mixing and un-mixing oil and water. A wide range of materials are compatible with STRIPS, and the method provides greater control of the shape that the final bijels take
Because we pull ethanol out directionally, the rate of bijel formation decreases from the outside to the inside of the material. That gives us structures with different length scales, such as intertwined fingers that are really fine on the surface but really open in the center. The multi-step nature of the phase separation additionally gives us nano-sized features with high surface areas. All of this helps us make what we call ‘asymmetric hierarchical structure’ which is helpful for size selective separation processes, amongst other applications.
Martin Hasse, Postdoctoral Researcher, Penn University
Bijels have complex internal structures meaning they could solve a problem in chemical reactions taking place in water; they also have products that are soluble in oil. "Emulsion microreactors” involve packing the reactants into water droplets with catalyzing particles on their surfaces, and then dipping them all in an oil bath.
The problem with this is that once you use up the reactants in your droplets, you’re done. All the droplets are isolated, so there’s no easy way to “restock” them with new reactants. That’s where bijels come in. You could keep on feeding the water phase with reactants and keep on pulling out product from the oil phase. We call that a ‘continuous reactor,’ and they’d be very useful for things like refining biofuels.
Daeyeon Lee, Associate Professor, Penn University
The team’s future work involves determining applications where STRIPS-formed bijels can be used.