Mar 9 2016
Metal-organic frameworks (MOFs) are low-cost materials that are capable of separating gases from air and other combined gas streams. When it comes to separating oxygen, these materials fail completely. To resolve this issue, researchers have developed a MOF composite and a helper molecule, where both work together to isolate oxygen from other types of gases in a simple and cost-effective way.
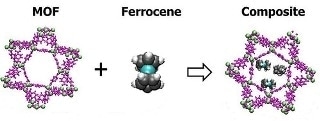 Squeezing iron-containing ferrocene (not to scale) in the pores of the metal-organic framework known as MIL-101 lets ferrocene's iron snag oxygen from passing air. (credit: PNNL)
Squeezing iron-containing ferrocene (not to scale) in the pores of the metal-organic framework known as MIL-101 lets ferrocene's iron snag oxygen from passing air. (credit: PNNL)
The study could be useful in many industrial processes such as; producing and using pure oxygen for fuel cells, developing oxygen sensors, eliminating oxygen in food packaging, etc. The findings have been published in Advanced Materials.
In addition to oxygen, the new method could be used with other gases by replacing the helper molecule. Currently, a standard process known as cryogenic distillation is utilized by the industry to isolate oxygen from other kinds of gases. However, this process is rather expensive and requires a large amount of energy to cool gases. It also cannot be used for specialty applications such as sensors, or for removing trace amounts of oxygen from food packaging. It would be easy to prepare and utilize an efficient oxygen separator, whether it is reusable or cheap. Plenty of pores are found in MOF materials that are capable of absorbing gases, similar to sponges absorbing water. MOFs could possibly be used in lightweight dehumidifiers and in nuclear fuel separation.
While countless MOFs are available only a few can take up molecular oxygen, and these viable MOFs react chemically with oxygen to form oxides, which make the material impractical and unfit for usage.
When we first worked with MOFs for oxygen separation, we could only use the MOFs a few times. We thought maybe there's a better way to do it.
Praveen Thallapally, Materials Scientist, Department of Energy's Pacific Northwest National Laboratory.
At PNNL Thallapally and his team applied a new approach, which involved the use of a helper molecule to mediate the separation of oxygen. While such a molecule would be attracted to the MOF, it would be chemically uninterested in it and instead would react with oxygen to isolate it from other gases.
MIL-101, a MOF material, was selected for the experiment as it has a high surface area and lacks reactivity. The high surface area makes the MIL-101 material a strong and powerful sponge, a teaspoon of MIL-101 exhibits the equivalent surface area as a football field. Such a high surface area is attributed to the pores in the MOF, where reactive MOFs work their best. In laboratories MOFs reacting with oxygen should be carefully handled, however MIL-101 remains stable in open atmospheres as well as in ambient temperature conditions. A low-cost, iron-containing molecule called ferrocene was used as a helper molecule.
The ferrocene and MIL-101 were combined together, and heated to produce a composite. Preliminary tests were performed which revealed that MIL-101 not only occupied more than its weight in ferrocene, but it also lost surface area simultaneously. This showed that the ferrocene took up space within the pores of MOF where they need to absorb the oxygen. Following this gases were allowed to pass via the black composite material, which trapped significant amounts of oxygen, yet practically none of the carbon dioxide, argon or, nitrogen gases that were added. Irrespective of whether the gases passed through separately or as a mix, the material behaved in this manner. This shows that the composite can isolate oxygen from other gases.
Further testing demonstrated that when ferrocene is heated up, it decomposes in the pores in the form of tiny, nano-sized clusters. As a result, the iron was available to react with oxygen.
This reaction produced maghemite, a stable mineral, within the pores of MOF. This mineral can then be extracted from the MOF so that it can be reused. The new results obtained on the composite indicate that MOFs could possibly be used to purify oxygen, with the aid of a helper molecule. The researchers are planning more research to study other combinations of helper molecules and MOFs.
In addition to the PNNL researchers, analytical instruments were used by participating scientists at two Office of Science User Facilities, the Advanced Photon Source at Argonne National Laboratory and the Environmental Molecular Sciences Laboratory at PNNL, and the University of Amsterdam.
The Department of Energy Office of Science funded the study.