Stability of protein formulations is critically important among the swiftly developing fields of protein therapeutics and vaccine production from recombinant antigens. Zeta potential, or the surface charge density at the shear plane, is one of the variables utilized in forecasting the stability of these preparations.
Moreover, zeta potential has been utilized as an indicator of conformational transformations in response to ligand addition. Conventional approaches to zeta potential quantification have implemented classical laser Doppler velocimetry to derive the electrophoretic mobility of suspended proteins.
Results from the phase analysis method, implementing an electrode design that removes electro-osmotic artifacts, are exhibited herein. Utilizing this method, measurement of zeta potential under a remarkable array of solvent conditions, which includes conditions of near-physiological ionic strength, becomes possible. This technique thus greatly enhances the scope of experimental conditions under which zeta potential can be established.
Experimental
Zeta potential quantifications were undertaken with a Brookhaven Instruments ZetaPALS. For improved sensitivity, the motion of the charged protein was probed with a technique known as phase analysis light scattering (PALS). In conventional laser Doppler electrophoresis, the frequency shift in the scattered light that occurs as a result of the motion of the scatterers is utilized to establish the proteins’ motion.
However, by identifying and quantifying the phase shift in the scattered light, it becomes possible to make far more sensitive measurements of particle motion.1 The optical arrangement is exhibited in Figure 1.
For extra sensitivity, an Uzgiris type electrode assembly, demonstrated in Figure 2, was utilized during these procedures. This design inhibits the electro-osmosis effect occurring in capillary type cells. Electro-osmosis is the bulk motion of a fluid due to an applied field and is a consequence of wall effects.
This bulk motion needs to be kept to a minimum as it impedes instrument sensitivity. The charged protein experiences motion in response to the applied field. Quantifications were executed at 25oC. Proteins were acquired from Sigma Aldrich and utilized as received.
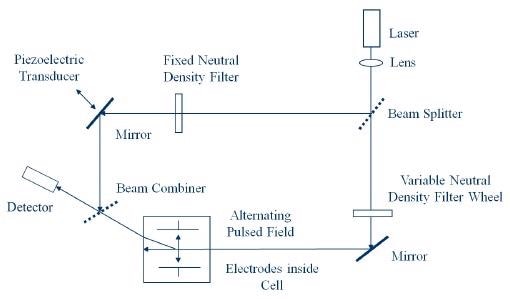
Figure 1. Optical Arrangement for Zeta Potential Determination.
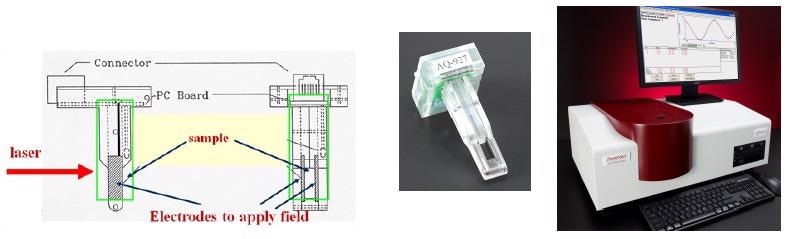
Figure 2. Uzgiris Cell for Zeta Potential Determination.
Theory
As it corresponds with the charge on the hydrodynamic unit, zeta potential, ζ , holds the highest intuitive appeal in regards to considerations of the surface charge. Nevertheless, the amount calculated by electrophoretic light scattering is electrophoretic mobility, µep. The phase shift of the scattered light, Φ, is established via measurement. It can subsequently be correlated to the mobility using the following equation.1
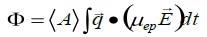
Here <A> represents the mean scattered amplitude, q represents the scattering vector the magnitude of which is (4πη/λ)sin(θ/2), where n is the refractive index of the medium, λ represents the wavelength of the light utilized to probe the sample, and θ represents the scattering angle. E represents the applied electric field. The integration is executed across the time span of the measurement. Mobility is converted to zeta potential with the use of a model. The selection of model is dependent on two variables, the double layer thickness and the particle size.
Ordinarily, the Smoluchovski model is suitable for aqueous solutions and the Huckel model for solutions that are non-aqueous. In this measurement, the Smoluchovski model, displayed below, is implemented to convert established mobility values to zeta potentials.
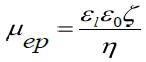
Here, εi represents the permittivity of the liquid, ε0 represents the permittivity of vacuum, and η represents liquid viscosity.
Results
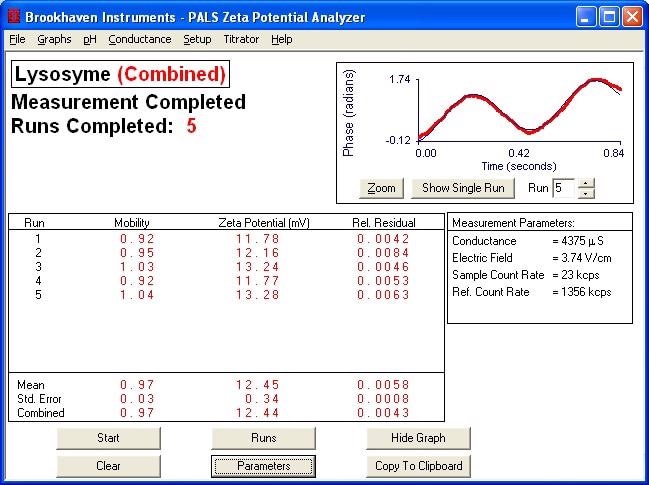
Typical Plot of Phase vs. Time for Phase Analysis
Zeta Potential of Various Proteins
| Protein (in PBS) |
Measured Zeta Potential (mV) |
| Lysozyme from Chicken Egg White |
5.91 |
| Apoferritin from Horse Spleen |
-13.70 |
| Bovine serum albumin, initial fraction |
-14.27 |
Effect of Salt Content on Lysozyme Zeta Potential. Note that decreased salt content leads to higher zeta potential values
| PBS concentration |
Measured Zeta Potential (mV) of Lyzsozyme |
| 1x |
5.91 |
| 0.1x |
12.45 |
Conclusion
The ZetaPALS can be implemented to establish the zeta potential of proteins in high salt suspension. Reduced salt content engenders increased zeta potential values as a result of the lowered impact of electrostatic shielding.
References and Further Reading
- Mobility Measurements by Phase Analysis, Walther W. Tscharnuter, Applied Optics, 40(24), August 2001, 3995-4003

This information has been sourced, reviewed and adapted from materials provided by Brookhaven Instrument Corporation.
For more information on this source, please visit Brookhaven Instrument Corporation.