Mar 21 2016
An innovative method for the stabilization of a multiply charged anion (negative ion) has been demonstrated by the researchers at New Virginia Commonwealth University. The study findings have the potential to help produce better magnesium and lithium ion batteries.
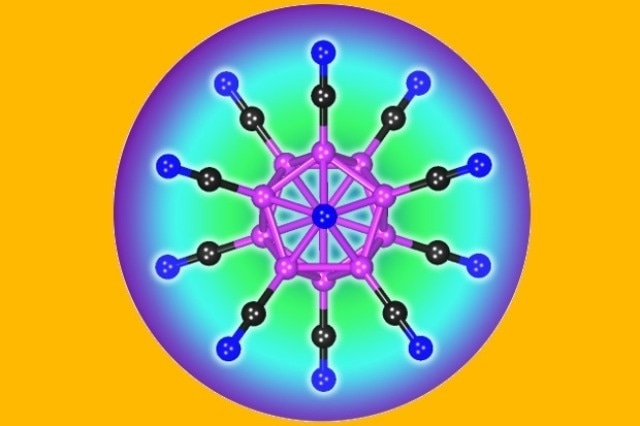 The structure of the B12(CN)122- dianion. This doubly charged negative ion holds the world’s record on stability.
The structure of the B12(CN)122- dianion. This doubly charged negative ion holds the world’s record on stability.
VCU physics researchers have described their work in Angewandte Chemie International Edition under the title “Stability of B12(CN)122-: Implications for Lithium and Magnesium Ion Batteries.” This paper has gained “Very Important Paper” distinction, demonstrating the significance of the research as less than 5% of papers published in this journal receive this status.
Multiply charged particles tend to be unstable in the gaseous phase. Electrons tend to repel one another when they are dumped in large numbers into a particle, leading to an unstable environment where the particle could break down, or it could eject electrons.
The VCU research team’s approach involves the substitution of ligands to generate a surprisingly stable, multiply charged molecule. Ligands are molecules or atoms that are capable of binding to a high-energy particle. This method also generated a molecule that behaves as both a dianion and monoanion.
This taught us that we can change ligands to effectively modulate chemistry. This gives us a very powerful tool to design a whole new class of complexes.
Puru Jena, Ph.D., Professor, Department of Physics of the College of Humanities and Sciences, New Virginia Commonwealth University
Hongmin Zhao, Ph.D., visiting professor from Beijing Jiantong University in China, and postdoctoral researcher Jian Zhou, Ph.D. have also contributed to the project.
The researchers began with a stable dianion, dodecaborate B12H122-, where the hydrogen atoms were substituted by CN molecules. The CN molecule consists of a carbon and nitrogen atom. An additional electron is required while placing C and N together, in order to form a closed-shell species that does not accept or release extra electrons.
This nonreactivity is crucial for stability, and Jena compares the closed-shell state to couples who are happily married.
If we are happy, we have no reason to do anything. But, if we are an unhappy, the reactions start. Once you close those shells, there’s no need for them to react by giving away or taking in new electrons because they are happy.
Puru Jena, Ph.D., Professor, Department of Physics of the College of Humanities and Sciences, New Virginia Commonwealth University
The newly developed multiply charged molecule, dodecacyanododecaborate B12(CN)122-, is a highly stable anion.
It holds the world’s record on stability, and there’s no other molecule that has been found that’s as stable as this one in a vacuum.
Puru Jena, Ph.D., Professor, Department of Physics of the College of Humanities and Sciences, New Virginia Commonwealth University
The researchers created another stable anion, CB11(CN)122-, by substituting boron atoms with carbon. This reaction was as expected, but what surprised the researchers was the resulting anion that showed stability comparable to a dianion.
If you replace boron with carbon, you only need one electron to close the shell. If you have two electrons, the shell is not closed. The molecule should be reactive and not take the extra electron. But it did.
Puru Jena, Ph.D., Professor, Department of Physics of the College of Humanities and Sciences, New Virginia Commonwealth University
This new complex was used by the researchers to design a halogen-free electrolyte for use in lithium and magnesium ion batteries, based on an earlier study focusing on safer, halogen-free batteries. Jena believes that the new research could help develop more efficient, more durable, and lighter-weight batteries.
The Fundamental Research Funds for Central University, the U.S. Department of Energy and CIT CRCF funded the research work.