Apr 25 2016
Unique and unanticipated behavior of water molecules under extreme confinement was revealed by neutron scattering and computational modeling. This behavior is unparalleled by any known solid, liquid, or gas states. A team of researchers at the Department of Energy’s Oak Ridge National Laboratory (ORNL) have illustrated a unique tunneling state of water molecules restricted in hexagonal ultra-small channels – 5Å across – of the mineral beryl. An angstrom is 1/10-billionth of a meter, and each atom is normally about 1Å in diameter. The findings have been published in Physical Review Letters.
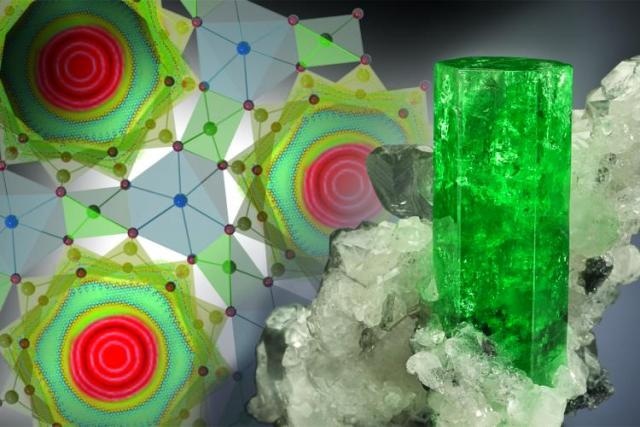 ORNL researchers discovered that water in beryl displays some unique and unexpected characteristics. (Photo by Jeff Scovil)
ORNL researchers discovered that water in beryl displays some unique and unexpected characteristics. (Photo by Jeff Scovil)
The researchers conducted experiments at ORNL’s Spallation Neutron Source and the Rutherford Appleton Laboratory in the UK, and illustrated characteristics of water under extreme confinement in soil, rocks, and cell walls, which researchers estimate will be of interest for several disciplines.
At low temperatures, this tunneling water exhibits quantum motion through the separating potential walls, which is forbidden in the classical world. This means that the oxygen and hydrogen atoms of the water molecule are ‘delocalized’ and therefore simultaneously present in all six symmetrically equivalent positions in the channel at the same time. It’s one of those phenomena that only occur in quantum mechanics and has no parallel in our everyday experience.
Alexander Kolesnikov, Chemical and Engineering Materials Division, ORNL
The presence of the tunneling state of water demonstrated that the ORNL research would enable researchers to better explain the thermodynamic properties and behavior of water in extremely restricted environments, such as in carbon nanotubes, along grain boundaries, water diffusion and transport in the channels of cell membranes, and at mineral interfaces in a number of geological environments.
ORNL researcher and co-author, Lawrence Anovitz stated that the discovery is ideal for initiating talks among geological, materials, biological, and computational researchers, as they try to understand the system behind this occurrence and comprehend how it can be applied to materials in their area of interest.
This discovery represents a new fundamental understanding of the behavior of water and the way water utilizes energy. It’s also interesting to think that those water molecules in your aquamarine or emerald ring – blue and green varieties of beryl – are undergoing the same quantum tunneling we’ve seen in our experiments.
Lawrence Anovitz, Researcher, ORNL
While preceding studies have noticed tunneling of atomic hydrogen in other mechanisms, the discovery by ORNL is unprecedented as water displayed such tunneling behavior. The experiments using neutron scattering and computational chemistry revealed that in the tunneling state the water molecules are delocalized around a ring, so the water molecule takes an abnormal double top-like form.
The average kinetic energy of the water protons directly obtained from the neutron experiment is a measure of their motion at almost absolute zero temperature and is about 30%t less than it is in bulk liquid or solid water. This is in complete disagreement with accepted models based on the energies of its vibrational modes.
Alexander Kolesnikov, Chemical and Engineering Materials Division, ORNL
Narayani Choudhury of Lake Washington Institute of Technology and University of Washington-Bothell made first principle simulations which illustrated that the tunneling behavior is linked to the vibrational dynamics of the beryl structure.
Co-authors of the paper, titled “Quantum Tunneling of Water in Beryl: a New State of the Water Molecule,” were Timothy Prisk, Eugene Mamontov, Andrey Podlesnyak, George Ehlers and David Wesolowski of ORNL, George Reiter of the University of Houston and Andrew Seel of Rutherford Appleton Laboratory. The research was funded by DOE’s Office of Basic Energy Sciences. The SNS is a DOE Office of Science User Facility.
UT-Battelle manages ORNL for the DOE's Office of Science. The Office of Science is the largest supporter of basic research in the field of physical sciences in the US, and is working to tackle certain highly pressing challenges faced by this generation.