May 17 2016
Fuel cells produce electricity from chemical reactions without any harmful emissions, and have the capability to power everything ranging from portable electronics to cars. They could be more clean and efficient, compared to combustion engines.
 Solid oxide fuel cells, which rely on low-cost ceramic materials, are among the most efficient and promising type of fuel cell. They work like batteries that don't need charging. (Image courtesy of WikiCommons)
Solid oxide fuel cells, which rely on low-cost ceramic materials, are among the most efficient and promising type of fuel cell. They work like batteries that don't need charging. (Image courtesy of WikiCommons)
Solid oxide fuel cells that depend on cheap ceramic materials are amongst the most competent and promising kind of fuel cell. Researchers at the Harvard John A. Paulson School of Engineering and Applied Sciences have discovered a way to gather the quantum behavior of the fuel cells in order to make them more effective and strong. While doing so, they have detected a different type of phase shift in an oxide material. The study has been published in the Nature journal.
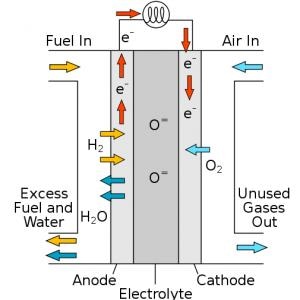
Fuel cells work in a similar way to batteries — producing an electric current by driving electrons to move between the anode and the cathode which are detached by an electrolyte. In contrast to batteries, fuel cells do not require recharge. All they need is fuel, often in the form of hydrogen.
When hydrogen is supplied to the anode, it splits to give rise to an electron and a proton. The electrolyte takes the role of a bouncer in an exclusive club – hindering electrons from entering and permitting protons to enter. The electrons are forced to take the longer route, via an external circuit, that generates a flow of electricity.
At the other side, air is supplied into the cell’s cathode. As the electrons move within the circuit and the protons move through the electrolyte, both bonding with the oxygen to generate water and heat, the sole emissions produced by the fuel cells.
Better fuel cells through quantum materials
However, the current solid oxide fuel cells have a main drawback. Over a period of time, the fuel reacts with the electrolyte and its efficiency is reduced. This permits both the electrons and protons through, making the electrical current weaker and weaker as it passes through the external circuit.
The answer to this problem has been found by Shriram Ramanathan, Visiting Scholar in Materials Science and Mechanical Engineering at SEAS, and his graduate student You Zhou. They found that by designing the electrolyte at the quantum level, a material can be produced that becomes more robust on exposure to fuel.
We have combined the fields of quantum matter and electrochemistry in a way that led to discovery of a new, high-performance material that can phase transition from a metal to ion conductor.
Shriram Ramanathan, Professor of Engineering, Purdue University
Ramanathan and his group made use of a nickelate that was perovskite-structured as their electrolyte. The nickelate conducts both ions and electrons by itself, similar to protons, making it a worthless electrolyte. However, the team covered the nickelate surface with a catalyst and then doped it with electrons. These electrons moved into the electron shell of the nickel ion and changed the material to an ion conductor from an electron conductor.
“Now, ions can move very quickly in this material while at the same time electron flow is suppressed,” said Zhou. “This is a new phenomenon and it has the potential to dramatically enhance the performance of fuel cells.”
The elegance of this process is that it happens naturally when exposed to the electrons in fuel. This technique can be applied to other electrochemical devices to make it more robust. It’s like chess — before we could only play with pawns and bishops, tools that could move in limited directions. Now, we’re playing with the queen.
Shriram Ramanathan, Professor of Engineering, Purdue University
The study was sponsored by Army Research Office, Air Force Office of Scientific, Research, Advanced Research Projects Agency-Energy (ARPA-E), IBM PhD Fellowship and National Academy of Sciences.