Oct 24 2016
 Chemists at Goethe University have now developed a new catalyst for the activation of hydrogen by introducing boron atoms into a common organic molecule. (Credit: GU)
Chemists at Goethe University have now developed a new catalyst for the activation of hydrogen by introducing boron atoms into a common organic molecule. (Credit: GU)
Chemists from Goethe University have developed a new catalyst to activate hydrogen by introducing boron compounds into a common organic molecule.
Hydrogen (H2) is a simple chemical element and a vital raw material as it is becoming more and more important in the development of sophisticated catalysts. It is widely used in industry and commerce, with applications ranging from fertilizer and food manufacture to crude oil cracking to deployment as an energy source in fuel cells.
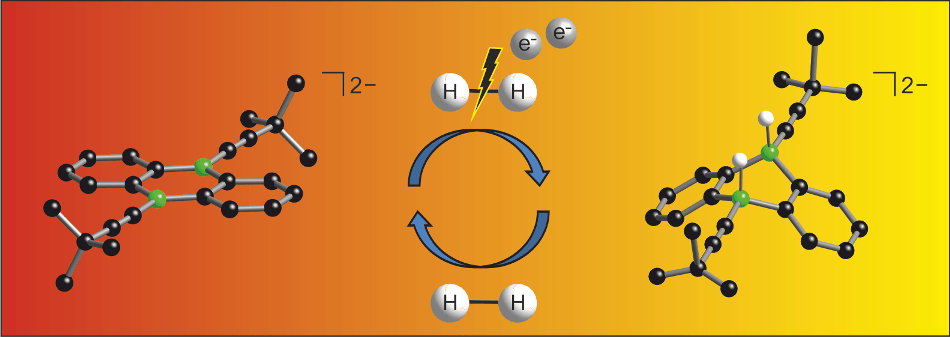
Splitting the solid H-H bond under mild conditions is challenging to researchers. This process solely requires an electron source and it should be used on a wide scale in future. The researchers process was described in the journal Angewandte Chemie.
The hydrogen molecule’s high energy content meets with a stable bonding situation. In 1897, Paul Sabatier detected for the first time that metals can be used as catalysts to split the molecule and harness simple hydrogen for chemical reactions. He received the Nobel Prize for Chemistry in 1912 for this important discovery.
Currently, the widely used hydrogenation catalysts contain expensive or toxic heavy metals, such as platinum, palladium and nickel. Non-metal systems based on phosphorous and boron compounds were discovered only ten years ago and they allow comparable reactions.
My doctoral researcher, Esther von Grotthuss, has achieved yet another major simplification of the non-metal strategy which requires only the boron component. What we additionally need is just an electron source. In the laboratory we chose lithium or potassium for this. When put into practice in the field, it should be possible to substitute this with electrical current.
Professor Matthias Wagner, Institute of Inorganic and Analytical Chemistry, Goethe University
In the study, quantum chemical calculations were performed in collaboration with Max Holthausen, a professor at Goethe University Frankfurt, to describe the intricacies of hydrogen activation above and over experimental findings. Detailed information of the reaction process is vital for further expansion of the system.
The objective of this strategy is not only replacing transition metals in a prolonged tenure but also to open the opportunity for chemical reactions that are not possible with standard catalysts.
The Frankfurt chemists believe that substitution reactions, in particular, are highly promising and allow easy access to hydrogen compounds with other elements. Expensive and potentially dangerous processes are still used for such syntheses. For instance, the simple production process of silicon-hydrogen compounds would be very appealing for the semiconductor industry.