Oct 6 2017
Scientists at the Department of Material Sciences, Lomonosov MSU, have described the manner in which the battery efficiency and structure of created films are influenced when the ratio of components that form light-absorbing layer of a perovskite solar cell is altered. The outcomes of the research have been reported in the Journal of Physical Chemistry C.
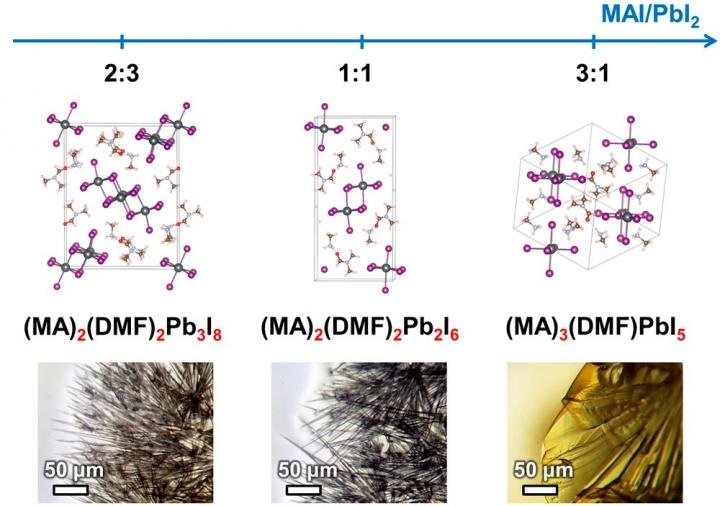 These are crystal structures of crystalline solvates. CREDIT: Alexey Tarasov.
These are crystal structures of crystalline solvates. CREDIT: Alexey Tarasov.
Organic-inorganic perovskites are an innovative type of photoactive materials—materials that react to light. They derive their name from the mineral known as perovskite—or calcium titanate, CaTiO3—as their structure is similar to perovskite. However, their original characteristics are far more impressive. By using these materials, we can develop perovskite solar batteries—introduced just half a decade ago. However, their efficiency is already far better than the more prevalent and very costly silicon solar elements.
In an earlier research, the Authors of the study discovered that filiform, or wire-like, hybrids of perovskites derived their shape from the structure of intermediate compounds formed through the course of perovskite crystallization. The team has found out a range of such compounds, where each compound is a crystalline solvate—a crystalline compound in which molecules of the solvent of its precursor components are formed into their structure. The dissolved components are precipitated from the solution to form a crystalline perovskite film.
The team chose and described three intermediate compounds that are crystalline solvates of one among two solvents mostly used in developing perovskite solar batteries. The crystal structure of two of the compounds was established for the first time.
We have found out that the formation of intermediate compounds is one of the key factors that determines functional properties of the final perovskite layer because perovskite crystals inherit the shape of those compounds. This, in turn, influences the film morphology and solar cell efficiency. It is especially important when creating thin perovskite films, because needle-like or filiform shape of crystals will lead to the film being discontinuous, which will significantly lower the efficiency of the solar cell. The knowledge on the influence of the ratio of precursor reagents on the shape of the final perovskite crystals will allow to deliberately choose the conditions for obtaining optimal films, which will result in perovskite cells with high efficiency.
Alexey Tarasov, PhD, the Principal Investigator of the study and Head of Laboratory of New Materials for Solar Energetics (Department of Materials Science, Lomonosov Moscow State University)
Intermediate compounds such as these are known to be unstable. Therefore, the team adopted synchrotron radiation as well as low temperatures to cool the crystals to nearly -173°C. Such cooling enabled them to arrest the decay of the crystals as well as to carry out the required measurements in order to ascertain the solvates’ structure.
Moreover, the team has investigated the thermal stability of the acquired compounds and was able to compute the energy of formation of the compounds by adopting quantum-chemical modeling. In-depth knowledge of the formation energy enables the Researchers to elucidate the formation of specific crystals upon using distinctive solvents.
The team also discovered that the nature of the intermediate compound formed during the crystallization process is particularly governed by the ratio of reagents in the solution. The shape of the formed perovskite crystals is governed by the crystal structure of the intermediate compound, and this shape in turn governs the structure of the light-absorbing layer. Consequently, this structure has an impact on the output of the developed solar battery.