Nov 8 2017
Researchers from the Department of Energy’s Oak Ridge National Laboratory and their colleagues have unearthed the fact that an “oxygen sponge”—a reliable catalyst, used in vehicle exhaust systems, with the ability to extract oxygen from air and store it for future use in oxidation reactions—can also be used as a “hydrogen sponge.” They have achieved this insight by adopting the right tool.
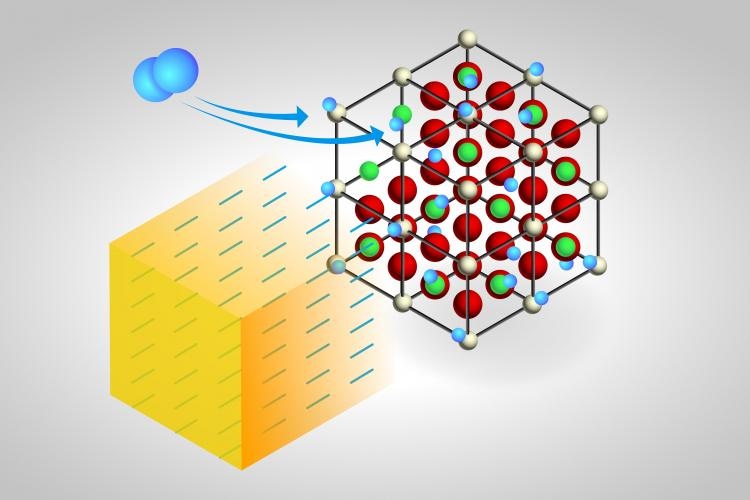 Neutrons probed two mechanisms proposed to explain what happens when hydrogen gas flows over a cerium oxide (CeO2) catalyst that has been heated in an experimental chamber to different temperatures to change its oxidation state. The first mechanism suggests hydrogen (H) atoms each associate with only oxygen (O) atoms to produce only OH species on the surface. The ORNL-led study instead provides evidence for the second mechanism, in which one H atom associates with an O atom to make OH and the other H associates with cerium (Ce) to make CeH—a hydride that may serve as a source of H for industrially important hydrogenation reactions. Color code: hydrogen, blue; oxygen, red; surface Ce, light yellow; bulk Ce, green. CREDIT: Oak Ridge National Laboratory, U.S. Dept. of Energy; illustrator Adam Malin.
Neutrons probed two mechanisms proposed to explain what happens when hydrogen gas flows over a cerium oxide (CeO2) catalyst that has been heated in an experimental chamber to different temperatures to change its oxidation state. The first mechanism suggests hydrogen (H) atoms each associate with only oxygen (O) atoms to produce only OH species on the surface. The ORNL-led study instead provides evidence for the second mechanism, in which one H atom associates with an O atom to make OH and the other H associates with cerium (Ce) to make CeH—a hydride that may serve as a source of H for industrially important hydrogenation reactions. Color code: hydrogen, blue; oxygen, red; surface Ce, light yellow; bulk Ce, green. CREDIT: Oak Ridge National Laboratory, U.S. Dept. of Energy; illustrator Adam Malin.
The outcomes of the research have been reported in the Journal of the American Chemical Society and can open the door for developing highly efficient catalysts for selective hydrogenation reactions. Selective hydrogenation is highly significant in synthesizing valuable chemicals — such as in selectively transforming triple-bonded hydrocarbons known as alkynes into double-bonded alkenes, which are the base materials for producing fuels, plastics and other commercial products.
Understanding how molecular hydrogen interacts with ceria [cerium oxide, CeO2], however, is a big challenge, as no regular technique can ‘see’ the light H atom. We turned to inelastic neutron spectroscopy, a technique that is very sensitive to hydrogen.
Zili Wu, Chemist, ORNL
At ORNL’s Spallation Neutron Source (SNS) — a DOE Office of Science User Facility — a neutron beam known as VISION investigated vibrational signals from atomic interactions and produced spectra that elucidated about them. “Because neutron spectroscopy could ‘see’ hydrogen due to its large neutron scattering cross-section, it succeeded where optical spectroscopy techniques failed and enabled the first direct observations of cerium hydrides both on the surface and in the bulk of a cerium oxide catalyst,” stated Wu.
In the case of vehicle engines, oxygen is required for the combustion of hydrocarbon fuel. The exhaust produced comprises of unburned hydrocarbons and harmful carbon monoxide. Inside the catalytic converter, cerium oxide (the catalyst) extracts oxygen from air and combines it with the hydrocarbons and carbon monoxide to transform them into carbon dioxide, which is harmless.
The discovery that cerium oxide has the ability to extract oxygen as well as hydrogen from air is favorable for attempts to manipulate it to catalyze not only reactions that result in electron gain, that is, “reduction” of a reactant, but also electron loss reactions, that is, “oxidation.”
The researchers have hypothesized two mechanisms to elucidate the reaction of molecular hydrogen with cerium oxide. According to one mechanism, both the hydrogen atoms get linked to oxygen atoms to synthesize the same product—two OH chemical groups, or hydroxyl species—on the surface. The other mechanism suggests that one of the hydrogen atoms gets linked with an oxygen atom to produce an OH group, whereas the other hydrogen atom gets linked with one cerium atom to produce cerium hydride, or CeH. The first mechanism is known as “homolytic,” and the second one is known as “heterolytic.”
The heterolytic reaction had not been seen before on cerium oxide. Theory predicted a heterolytic reaction, but there was no experimental proof.
Zili Wu, Chemist, ORNL
At the Center for Nanophase Materials Sciences (CNMS) (a DOE Office of Science User Facility at ORNL), the team synthesized nanoscale crystalline cerium oxide rods that had well-defined surface structure to achieve an in-depth knowledge of catalytic reactions that can be challenging to carry out with commercial, usually spherical particles of cerium oxide.
The nanoscale rods enabled the researchers to distinguish between hydrogen in the bulk and hydrogen on the surface, where catalysis was supposed to occur. The first discovery of hydrides on the surface as well as in the bulk of ceria was significant as it proved that the bulk of the material can also take part in the chemical reactions.
Moreover, at CNMS, Wu and Guo Shiou Foo conducted in situ experiments by adopting Raman and infrared spectroscopy, which involve scattering photons to form spectra that offer “fingerprints” of atomic vibrations. Regrettably, such optical methods “see” just the vibrating oxygen–hydrogen bonds, or stretching between the oxygen and hydrogen bonds. They cannot observe the hydride species on ceria.
For direct observation of the hydrogen interactions, the team had to make use of SNS, where Yongqiang Cheng, Luke Daemen and Anibal Ramirez-Cuesta carried out inelastic neutron scattering. Simultaneously, Franklin Tao, Luan Nguyen and Xiaoyan Zhang from the University of Kansas used the technique of ambient-pressure X-ray photoelectron spectroscopy to identify cerium oxide’s oxidation state — a highly crucial factor in deriving the mechanism.
Furthermore, Cheng, with assistance from Ariana Beste from the University of Tennessee, developed theory-based simulations of neutrons’ vibrational spectra and compared them with experimental observations. This collaboration played a vital role in gaining an in-depth knowledge of the reaction between cerium oxide-based catalysts and molecular hydrogen.
The present neutron study adopted VISION to investigate the properties of the hydride species in the catalyst. Future investigations will involve using another beam line called NOMAD to identify the precise structure of both the bulk and surface hydride in the catalyst to unearth, for instance, whether oxygen vacancies give rise to channels in the bulk to allow the entry of hydrogen and stimulate further formation of hydride.
A significant fact is that the team will exploit NOMAD’s potential to measure diffraction patterns at temperatures conducive for chemical reactions to occur. By adding hydrocarbons, the researchers will investigate and unearth the catalytic part played by the surface hydride against that of the bulk hydride in the case of hydrogenation reactions.
The knowledge gained will assist in developing more efficient cerium-based catalysts for a range of applications.
The paper is titled “Direct Neutron Spectroscopy Observation of Cerium Hydride Species on a Cerium Oxide Catalyst.”
The study was supported by the DOE Office of Science. ORNL’s Laboratory Directed Research and Development Program made the computing resources available.