Digicom Electronics, Inc., a technology and quality driven electronics manufacturing services company based in Oakland, CA, will present ways to mitigate device failure in manufacturing processes, at the BIOMEDevice Show, booth #327, December 6 and 7th, at the San Jose Convention Center in San Jose, CA. In addition, Digicom will be celebrating its 35th year in the California Bay Area.
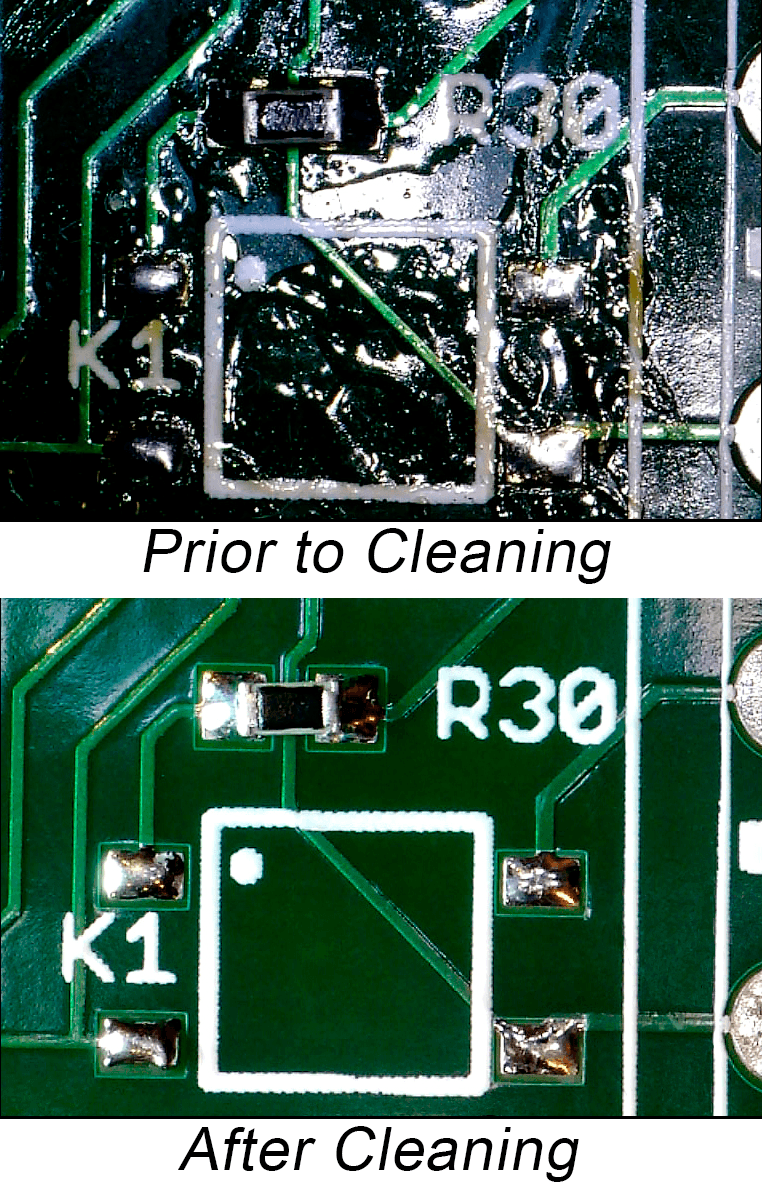
"Device failure can often be attributed to printed circuit board contamination or problems with the solder joints," explained Mo Ohady, general manager of Digicom Electronics. "Digicom has spent several years researching ways to produce ultra-clean boards and to build-in reliability throughout the assembly process. We developed a proprietary Diamond Track Cleaning Process, which independent testing agencies verify produces boards with zero ion contamination. In addition, studies show a 50-60% reduction in defect level when using nitrogen in the reflow process. Digicom generates its own nitrogen and pipes it to all our soldering processes, including hand, reflow, and selective soldering. 100% of our boards are inspected. We feel that it's essential that an EMS company take every step to ensure the integrity of the devices they prototype and assemble."
Digicom helps companies with their complete process from design review through prototyping, component sourcing, manufacture, test, and process validation. It has been recognized as one of the first companies to have been certified for the new AS9100:2016 (Rev D) aerospace quality and ISO 9001:2015 standards, and has ISO 13485:2003 medical devices quality, quality system regulation 21 CFR 820, ITAR, and numerous other certifications. See our videos, articles, and information here, follow us on twitter here, call +1.510.639.7003, or e-mail [email protected].