Mar 30 2018
A new method to create self-assembled biomaterials that depend on protein modifications and temperature has been demonstrated by biomedical engineers from Duke University. The hybrid method allows the researchers to regulate self-assembly more precisely, which may prove beneficial for several biomedical applications from drug delivery to wound healing.
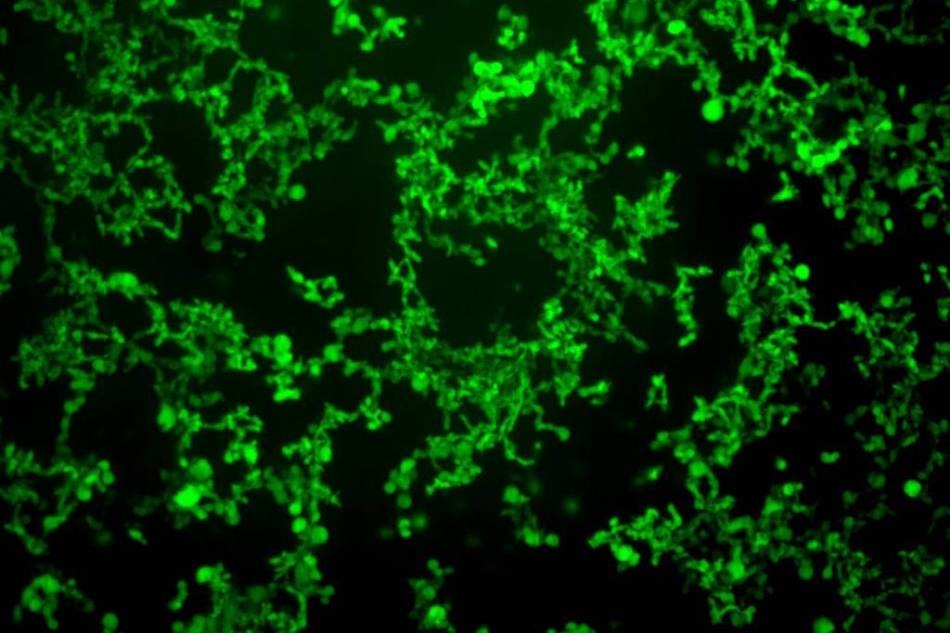 Self-assembled structures formed by fatty-acid-modified elastin-like polypeptides (FAME). Researchers use temperature cues to make the molecules assemble themselves. (Image credit: Davoud Mozhdehi and Kelli Luginbuhl)
Self-assembled structures formed by fatty-acid-modified elastin-like polypeptides (FAME). Researchers use temperature cues to make the molecules assemble themselves. (Image credit: Davoud Mozhdehi and Kelli Luginbuhl)
The study has been published online on March 19 in Nature Chemistry.
Biomaterials have extensive applications spanning the fields of tissue engineering, drug delivery, and regenerative medicine. Protein- and peptide-based materials are appealing for these applications as they are biodegradable, non-toxic, and have a distinct composition. But these biomaterials are restricted to the 20 amino acids originating in nature.
One approach to increase the chemical diversity of protein-based materials is a post-translational modification (PTM), a strong set of reactions that nature uses to chemically change proteins after they are synthesized from genes. PTM can alter particular amino acids in proteins or incorporate non-protein structures, such as fatty acids and sugars.
Nature combines different chemical alphabets to make very sophisticated materials. One way it does this is by combining the amino acid vocabulary of proteins with other very different alphabets -- sugars and fats are just two examples of the many hundreds of such PTMs. As materials scientists, we have not taken advantage of nature’s methods to make hybrid materials, and this provided the inspiration for this research.
Ashutosh Chilkoti, Author & Chair of the BME department at Duke
To create such a hybrid material with beneficial biomedical properties, researchers in the Chilkoti lab concentrated on forming a series of lipid-modified polypeptides, also called fatty-acid-modified elastin-like polypeptides (FAMEs).
When a lipid is fused to a peptide sequence, the various physical properties of the lipid and peptide cause the formation of peptide amphiphiles or PAs. Typical PAs can self-assemble into varied structures like long fibers, making them beneficial as scaffolds for tissue engineering. However, this occurs spontaneously and it is not possible to inject these materials into the body. They can only be implanted.
The team added another valuable biomaterial, elastin-like polypeptide (ELP), as it can transform from a soluble state to an insoluble state, or vice-versa, subject to temperature.
Using three components - a lipid myristoyl group‚ a beta-sheet-forming peptide sequence, and an elastin-like polypeptide (ELP) - the researchers developed a hybrid biomaterial, the FAME polypeptide that transforms from molecules floating in solution into a solid material, just by increasing the temperature.
Attachment of lipids to a short sequence of peptides, typically 5-20 amino acids, have been investigated for many years, but combining large biopolymers with lipids had not been explored. What distinguishes FAMEs from PAs is the presence of this temperature-sensitive biopolymer with much longer length, typically 200-600 amino acids, in the form of the ELP.
Davoud Mozhdehi, Postdoctoral Fellow - Chilkoti lab
“That short beta sheet-forming peptide sequence only makes up about two percent of the entire sequence,” Mozhdehi said. “But it has a huge impact on the self-assembly behavior. This hybrid material retains thermal responsiveness of the ELP and the hierarchical self-assembly of the PA, creating a unique material with programmable behavior.”
“By combining a PA with an ELP, we get a molecule that can go from liquid to solid within seconds with a small rise in temperature”, said Chilkoti. “This opens up new applications in medicine, whereas these materials can be injected as a liquid that would then turn into solid inside the body.”
This proof-of-concept takes off from an earlier research from the Chilkoti lab, where researchers studied new ways to apply enzymes to synthesize hybrid lipid-peptide polymer fusions between ELPs and lipids using E. coli bacteria.
Others had previously found that you can take a specific enzyme out of complex eukaryotic cells and get it to function in E. coli. Normally, this enzyme permanently attaches a lipid group to a protein, and we were curious whether we could use the enzyme to make lipid-biopolymer hybrid materials. When Davoud Mozhdehi heard about this project, he had an idea to incorporate a short structure-directing peptide sequence into the mix.
Kelli Luginbuhl, Research Scientist - Chilkoti lab
Researchers at the Max Planck Institute for Polymer Research helped the Duke team by concluding advanced material characterization. “Upon hearing about the multiple structures formed by these bio-manufactured polymers, we were quite excited to participate in this collaborative project to further elucidate the mechanism of temperature-triggered hydrogel and aggregate formation in these materials,” the Max Planck team said in a statement. “Our contribution of temperature-dependent, high-resolution atomic force microscopy and temperature-dependent spectroscopy nicely complemented the work from the Duke group, and together we were able to decipher the molecular transformations by which these unique biopolymers form hierarchical materials.”
“These building blocks are known in the field and now we have shown that combining them by forming covalent bonds, results in synergistic properties and self-assembly,” Mozhdehi said. “We hope to expand this method to other lipids and proteins and develop new tools and materials for the biomedical applications.”
This study received funding from the National Science Foundation through the Research Triangle Materials Research Science and Engineering Center (MRSEC; DMR1121107), National Institutes of Health (NIH, R01 GM-061232). Duke University Shared Materials Instrumentation Facility and Analytical Instrumentation Facility at North Carolina State University are members of the North Carolina Research Triangle Nanotechnology Network, which is supported by the NSF (ECCS-1542015) as part of the National Nanotechnology Coordinated Infrastructure.