Feb 6 2019
Polyvinylidene fluoride, or PVDF in short, is a ferroelectric polymer that possesses a number of unique properties and can possibly be used for storing energy or information.
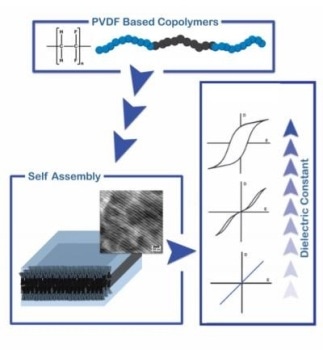 A block copolymer of PVDF (black) and an insulating polymer chain (blue) form a block copolymer. Through phase separation, the blocks assemble in films. The dielectric properties can be tuned by varying composition and length of the blocks. (Image credit: Department of Polymer Science, University of Groningen)
A block copolymer of PVDF (black) and an insulating polymer chain (blue) form a block copolymer. Through phase separation, the blocks assemble in films. The dielectric properties can be tuned by varying composition and length of the blocks. (Image credit: Department of Polymer Science, University of Groningen)
However, PVDF has one major disadvantage—when additional functional groups are added to enhance specific properties, its ferroelectricity is affected. In order to overcome this problem, researchers from the University of Groningen have developed block copolymers from PVDF that enable them to adjust its properties without interfering with its ferroelectricity.
In addition to studying how this ferroelectric polymer works, the researchers also wanted to broaden its application to include flexible organic electronics. The study results have been reported in the journal Nature Communications on February 6th, 2019.
PVDF polymers have polar structures with dipoles that can be easily aligned upon applying an electric field. By altering the direction of the electric field, it is possible to reverse the orientation of the dipoles. Therefore, the material displays switchable behavior, implying that it can possibly be used for storing information. Due to the existence of dipoles in the PVDF polymer and its high dielectric constant, energy storage in capacitors may also provide an option, albeit its ferroelectricity would lower the efficiency of these capacitors.
Phase separation
This problem could be solved by modifying the material.
However, modifying the molecules by attaching side chains affects their ferroelectric properties.
Ivan Terzic, Study Co-First Author and PhD Student, Department of Polymer Science, University of Groningen.
In association with Niels Meereboer, his fellow PhD student; and Katja Loos, their supervisor and Professor of Polymer Science, Terzic developed a method to create a copolymer of trifluoroethylene and vinylidene fluoride that has a functionalized end group, which can be connected to an insulating polymer chain to create a block copolymer. The researchers then demonstrated that minute domains at nanometer scales are formed by the material, via phase separation between the blocks. These small domains take varied shapes, for example, spherical, cylindrical, or lamellar, based on the ratio between the blocks.
Free-standing films
Terzic further informed that “Others have tried to prepare PVDF block copolymers, but they could only produce blocks with short polymer chains. In that case, the blocks mix and show no phase separation.” By changing the block type and preparing block copolymers of sufficient length, the researchers successfully tuned the material’s properties. A significant part of this study was the potential to make free-standing polymer films that have adequate mechanical characteristics. “This allowed us to investigate the properties of the material,” added Terzic.
In addition, Terzic applied block copolymers in order to enhance the interplays between PVDF and inorganic nano-objects and also to enhance their dispersion within the polymer. Magnetic nanoparticles, for instance, can be added to the PVDF to create a multiferroic material that possesses ferromagnetic as well as ferroelectric properties, implying that it can be combined together. In addition, changing the PVDF’s behavior could allow the energy to be retrieved in a more efficient manner.
“That would allow us to make a highly efficient capacitator that could be used wherever stored energy needs to be released fast, like in defibrillators or to convert direct current from solar panels to alternating current,” Terzic stated.
Toolbox
On the whole, the authors have developed a toolbox to produce varied types of PVDF-based block copolymers that have tunable properties.
We can use this to increase our understanding of the ferroelectric and other properties of PVDF, but also for new applications. The organic PVDF is flexible, lightweight and non-toxic, in contrast to some inorganic ferroelectrics that often contain lead. And it is bio-compatible, so medical applications are another interesting possibility.
Ivan Terzic, Study Co-First Author and PhD Student, Department of Polymer Science, University of Groningen.