Feb 15 2019
Researchers at Rice University are taking the next step toward the deployment of strong, rechargeable lithium metal batteries by rendering them simpler and safer to manufacture.
 A layer of red phosphorus in rechargeable lithium metal batteries can signal when damaging dendrites threaten to create a short circuit. The technique developed at Rice University could lead to more powerful lithium metal batteries. (Courtesy of the Tour Group)
A layer of red phosphorus in rechargeable lithium metal batteries can signal when damaging dendrites threaten to create a short circuit. The technique developed at Rice University could lead to more powerful lithium metal batteries. (Courtesy of the Tour Group)
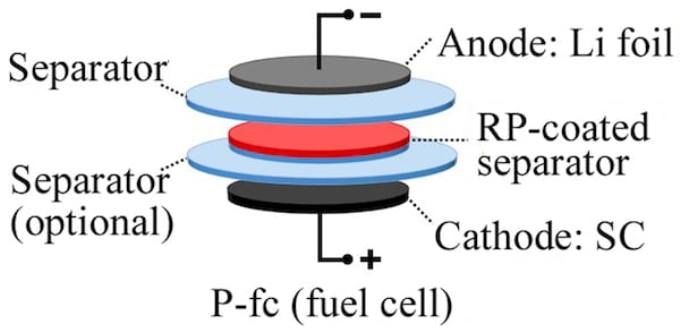
The Rice lab of chemist James Tour created test cells with a red phosphorus coating on the separator. The separator keeps the cathode and anode electrodes away from each other. The phosphorus serves as a spy for management systems used to charge and track batteries by sensing the development of dendrites, lithium protrusions that can cause them to be faulty.
Lithium metal anodes charge a lot faster and store almost 10 times more energy by volume than regular lithium-ion anodes used in nearly every commercial electronic gadget, including electric cars and cellphones. Anodes are one of two electrodes required for operating a battery.
But charging lithium-infused anodes causes the development of dendrites that, if they come in contact with the cathode, result in a short circuit and probably a fire or explosion. When a dendrite comes in contact with a red phosphorus-coated separator, the battery’s charging voltage changes. That alerts the battery management system to discontinue charging.
In contrast to other suggested dendrite detectors, the Rice strategy does not require a third electrode.
Manufacturing batteries with a third electrode is very hard. We propose a static layer that gives a spike in the voltage while the battery is charging. That spike is a signal to shut it down.
James Tour, T.T. and W.F. Chao Chair in Chemistry, and Professor of Computer Science and of Materials Science and Nanoengineering, Rice University.
The study has been published in Advanced Materials.
The experiments on test batteries by the Tour lab revealed no major effect was observed on regular performance due to the use of the red phosphorus layer.
The scientists constructed a transparent test cell with an electrolyte (the gel-like or liquid material between the electrodes and around the separator that permits the battery to produce a current) known to hasten aging of the cathode and promote dendrite growth. That allowed them to monitor the voltage while they watched dendrites develop.
With a normal separator, they observed the dendrites reach and penetrate the separator with no change in voltage, a condition that would lead a regular battery to malfunction. But with the red phosphorus layer, they noted a clear drop in voltage when the dendrites touched the separator.
“As soon as a growing dendrite touches the red phosphorus, it gives a signal in the charging voltage,” Tour said. “When the battery management system senses that, it can say, ‘Stop charging, don’t use.'”
Last year, the lab presented carbon nanotube films that seem to totally stop dendrite growth from lithium metal anodes.
By combining the two recent advances, the growth of lithium dendrites can be mitigated, and there is an internal insurance policy that the battery will shut down in the unlikely event that even a single dendrite will start to grow toward the cathode. Literally, when you make a new battery, you’re making over a billion of them. Might a couple of those fail? It only takes a few fires for people to get really antsy. Our work provides a further guarantee for battery safety. We’re proposing another layer of protection that should be simple to implement.
James Tour, T.T. and W.F. Chao Chair in Chemistry, and Professor of Computer Science and of Materials Science and Nanoengineering, Rice University.
Rice graduate student Tuo Wang is the paper’s lead author and postdoctoral researcher Rodrigo Salvatierra is the co-author. Tour is the T.T. and W.F. Chao Chair in Chemistry as well as a professor of computer science and of materials science and nanoengineering at Rice.
The research was supported by the Air Force Office of Scientific Research.