The Vocus PTR-TOF routinely detects a very large number of VOCs, including many for which standards are not available. It is possible to calibrate for all detected compounds with good accuracy based on simple measurements of a small number of VOC standards.

Felipe Lopez-Hilfiker, Liang Zhu, Manuel Hutterli, Luca Cappellin
TOFWERK, Thun, Switzerland
Quantifying High Numbers of VOCs Requires an Efficient Strategy to Calibrate PTR-TOF Sensitivity
The ability to quantify analytes based on direct calibration of a subset of standards is a major advantage of chemical ionization mass spectrometry (CI-MS). This is particularly important for CI-MS that use time-of-flight (TOF) mass analyzers, which can simultaneously measure thousands of compounds.
The advent of the ultra-high sensitivity Vocus PTR-TOF led to a significant increase in the number of volatile organic compounds (VOCs) that can be routinely detected.1 Many of these VOCs are highly reactive or are not readily available as standards. Further, it is not practical to independently calibrate PTR-TOF sensitivity for each detected VOC. Quantitative interpretation of the data thus requires a method to convert recorded signal intensities to concentrations without direct calibration of each compound.
For any analyte molecule, combined knowledge of its proton transfer rate constant, kPTR, and the instrument response to VOC standards at the utilized operating conditions allows straightforward determination of the Vocus PTR-TOF sensitivity for that molecule. Calculated sensitivity scalars can be used to convert recorded mass spectral signal intensities to more meaningful concentration values.
Measuring the Linear Relationship between Rate Constants and PTR-TOF Sensitivity
By analyzing a gas standard containing VOCs with different molecular weights and known reaction rate constants, one can determine the linear relationship between Vocus PTR-TOF sensitivity and proton transfer rate constants, kPTR, at the fixed, known operating conditions. An example of such a relationship is shown in Figure 1. This procedure gives a first estimate of the sensitivity for a compound having known kPTR without use of a standard. Accurate calculation of sensitivity must also take into account mass dependent processes in the mass spectrometer that affect observed signal intensities.
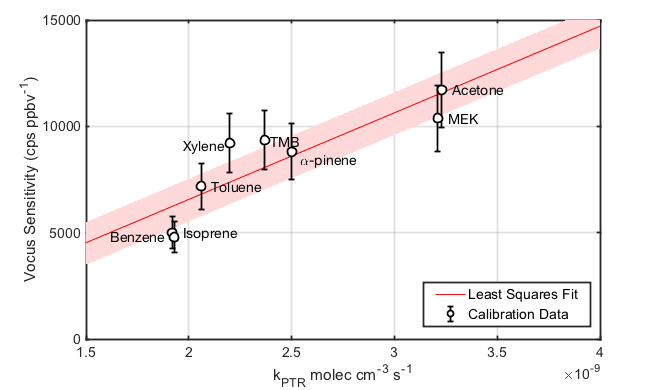
Figure 1 Sensitivity of Vocus PTR-TOF as a function of analyte proton transfer reaction rate constants, kPTR. The linear relationship enables the calculation of sensitivity for compounds having known kPTR without use of a standard.
Measuring the Mass Transmission Curve of the TOF Mass Spectrometer
To calibrate Vocus PTR-TOF sensitivity for an arbitrary analyte based on the response of the instrument to different standard molecules, one must consider how the masses of the various analytes affect their relative sensitivities. A mass transmission curve describes the mass-dependent detection efficiency of the mass spectrometer. For the Vocus PTR-TOF, this curve is a function of various parameters, including the bandpass windows of ion guides, the TOF extraction processes, and discrimination effects during MCP detection. It is not possible to accurately calculate this complicated function, so it is best measured. Vocus PTR-TOF calibration data, such as those displayed in Figure 1, can also be used for this purpose.
Figure 2 shows a typical mass transmission curve, with the y axis representing the fraction of ions transmitted from the source to the detector (recorded ions/total ions). For each analyte used to generate such a curve, the total ion value can be calculated based on its proton transfer reaction rate constant.
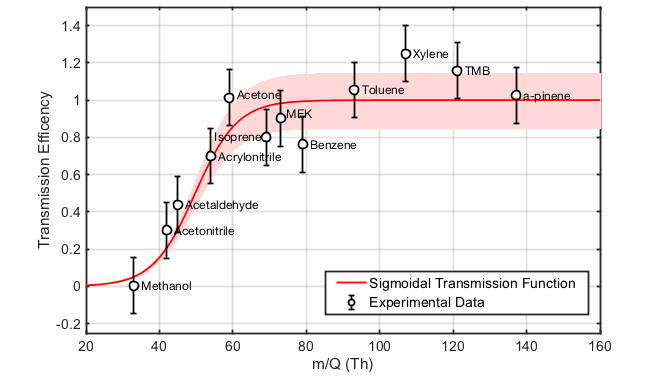
Figure 2 Mass transmission curve of a Vocus PTR-TOF determined by measurement of a gas calibration standard. The bandpass of the interface ion guide is set to remove the high intensity reagent ions, H3O+ (19 Th) and H2OH3O+ (37 Th), from the spectrum. This effect combines with the TOFs reduced duty cycle to yield the observed low transmission at low m/Q (20-50 Th). Above this, the mass transmission of the instrument is approximately constant to several hundred Th, before gradually falling at m/Q > 500. As described in the text, the transmission curve is important for calculating sensitivities for compounds not directly calibrated, but for which a reaction rate constant is known or can be calculated.
Calibrating PTR-TOF Sensitivity for a VOC without a Standard
Having measured the Vocus PTR-TOF sensitivity and mass transmission curve for a basis set of compounds, it is then straightforward to calculate the sensitivity, Sobs, for a VOC of known kPTR. In particular, the sensitivity value for a given VOC is the product of the sensitivity predicted based on the measured relationship between sensitivity and rate constant, f(kPTR), and the transmission efficiency at the VOC’s mass/charge, Trans[m/Q].
Sobs = Sens[f (kPTR) ]×Trans[m/Q]
The concentration of the VOC is then calculated as the recorded signal (counts per second, cps) divided by the calculated sensitivity.
[VOC]= SignalVOC (cps) / Sobs
Using this approach, it is possible to quantify compounds for which the reaction rate constant (kPTR) is known within a typical accuracy of +/- 30%.
For a compound for which no reaction rate constant is available, it is possible to calculate a semi-quantitative concentration using an average reaction rate coefficient or an approximation based on molecular properties.2 Because PTR reaction rates are nearly collision limited3 and reactor conditions are precisely controlled, the normal span of PTR reaction rates is in the range of 2-3.5 x 10-9 cm3molecules-1s-1. The error introduced by such an assumption is thus significantly smaller than in other ionization approaches. Without calibration, measurement accuracy for analytes having unknown rate constants can often be better than 50%, assuming no extensive fragmentation upon ionization.
The high sensitivity Vocus PTR-TOF routinely detects a very large number of VOCs, including many for which standards are not available. Utilizing well established reaction kinetics and precise control of reaction conditions, it is possible to calibrate PTR-TOF sensitivity for all detected compounds with good accuracy based on simple measurements of a small number of VOC standards.
References
1. Riva, M.; Rantala, P.; Krechmer, J. E.; Peräkylä, O.; Zhang, Y.; Heikkinen, L.; Garmash, O.; Yan, C.; Kulmala, M.; Worsnop, D.; and Ehn, M. Evaluating the performance of five different chemical ionization techniques for detecting gaseous oxygenated organic species. Atmos. Meas. Tech. Discuss., 2018, In Review. https://doi.org/10.5194/amt-2018-407
2. Sekimoto K; Li, S. M; Yuan, B; Koss, A; Coggon, M; Warneke, C; de Gouw, J. Calculation of the sensitivity of proton-transfer-reaction mass spectrometry (PTR-MS) for organic trace gases using molecular properties. Int. J. Mass Spec. 2017, 421, 71-94. https://doi.org/10.1016/j.ijms.2017.04.006
3. de Gouw, J.; Warneke, C.; Measurements of volatile organic compounds in the earth’s atmosphere using proton‐transfer‐reaction mass spectrometry. Mass Spec Rev. 2006, 26, 223–257. https://doi.org/10.1002/mas.20119