Jun 7 2019
Blending two additives rather than one to enable the integration of lithium within capacitors is the solution put forward by scientists from l’Institut des matériaux Jean Rouxel (CNRS/Université de Nantes), together with Münster Electrochemical Energy Technology (University of Münster, Germany), in order to advance the low-cost, uncomplicated, and efficient development of the lithium-ion capacitors employed to store electrical energy.
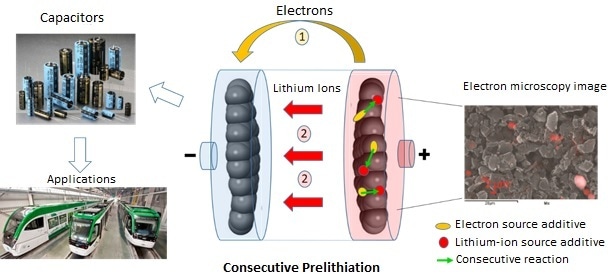 Consecutive prelithiation involving two additives (pyrene in yellow and Li3PO4 in red). The chemical analysis used in the electron microscopy image makes it possible to locate Li3PO4 (red areas). (Image credit: Joel Gaubicher, Institut des matériaux Jean Rouxel (CNRS/Université de Nantes))
Consecutive prelithiation involving two additives (pyrene in yellow and Li3PO4 in red). The chemical analysis used in the electron microscopy image makes it possible to locate Li3PO4 (red areas). (Image credit: Joel Gaubicher, Institut des matériaux Jean Rouxel (CNRS/Université de Nantes))
This study, reported in Advanced Energy Materials on June 5th, 2019, will allow the mass marketing of these components.
Electrochemical storage systems for electricity play an important role in the incorporation of renewable energy sources, and are ready to take over the electro-mobility sector. This energy can be stored in two ways: lithium-ion batteries, which have the benefit of large storage capacity, and capacitors, which have less capacity, but can charge and uncharge very quickly for a large number of times. Lithium-ion capacitors (LIC) incorporate the best of both worlds.
In contrast to batteries, the materials that constitute lithium-ion capacitors do not possess lithium ions (or electrons). It is thus essential to continue with a prelithiation phase in order to incorporate them, so that the device can operate. At present, two wide approaches are used: either one of the capacitor’s constituent materials is prelithiated prior to its integration, or an additive high in lithium ions will redistribute them among the capacitor’s materials during the first charge. However, these techniques are expensive and complex, and can reduce the device’s capacity. Furthermore, most of the prelithiation additives available degrade upon contact with the air and/or the solvents used to produce lithium-ion capacitors. In a nutshell, although some of the solutions that have been projected work currently, there is no “miracle recipe” that is sturdy, simple, inexpensive, and offers high-performance.
Scientists from l’Institut des matériaux Jean Rouxel (CNRS/Université de Nantes), together with Münster Electrochemical Energy Technology (University of Münster), overcame this issue by using two additives rather than one coupled through successive chemical reactions. Their analysis indicates that the main hindrance for former methods was their use of just one additive, which had to supply lithium ions and electrons and also had to satisfy all of the conditions of chemical stability, price, and performance.
The use of two additives each with a particular role, with one offering lithium ions and the other offering electrons, provides much greater liberty, as they can be chosen independently for their chemical properties, performance, and price. When a lithium-ion capacitor is charging, the first additive (pyrene, naturally found in certain kinds of coal) discharges protons and electrons. The second additive, Li3PO4 (mass produced in the glass industry, for instance), traps these protons, and in turn discharges lithium ions that are then available for prelithiation.
An added benefit of this technique is that after prelithiation, pyrene—the residue of one of the two additives used—contributes to the storage of charges, thus increasing the amount of electrical energy stored in the device. The versatility and efficiency provided by this new technique pave the pathway for an economical solution for prelithiation, leading to lithium-ion capacitors that can store more energy. The breaking of this technological hurdle should hence allow a more rapid commercialization of these devices.