Jun 25 2019
The long list of targets of Green chemistry includes two well-studied ones in particular: production of hydrogen from renewable resources and conversion of biomass into fuels and useful chemicals.
 When irradiated by visible light, this catalyst converts biomass compounds to diesel-fuel precursors and hydrogen. (Image credit: Nature Energy)
When irradiated by visible light, this catalyst converts biomass compounds to diesel-fuel precursors and hydrogen. (Image credit: Nature Energy)
Although most research focus on either one subject or the other, an international research team has currently reported a two-for-one deal: an approach to generate hydrogen and diesel-fuel precursors from the starting materials derived from biomass. The study has been published in Nature Energy.
The breakdown of water into oxygen and molecular hydrogen—in the presence of a photocatalyst and sunlight—could lead to an appealing and cheap method for generating clean-burning hydrogen fuel, which often depends on the semiconductor catalysts. These catalysts produce excited electrons upon illumination and reduce protons in water to generate hydrogen.
However, the absorption of light can also generate excited holes or positive charge carriers. These strong oxidizers can eventually destroy the catalyst and reduce its effectiveness. Scientists scavenged these damaging charges by often adding sacrificial reagents. Although this approach maintains high rates of hydrogen production, scavengers produced waste products at high cost.
Instead of removing the holes to prevent them from causing problem, Nengchao Luo and Feng Wang from the Dalian Institute of Chemical Physics and colleagues aimed at using these species to induce reactions that are helpful in converting biomass compounds into something useful.
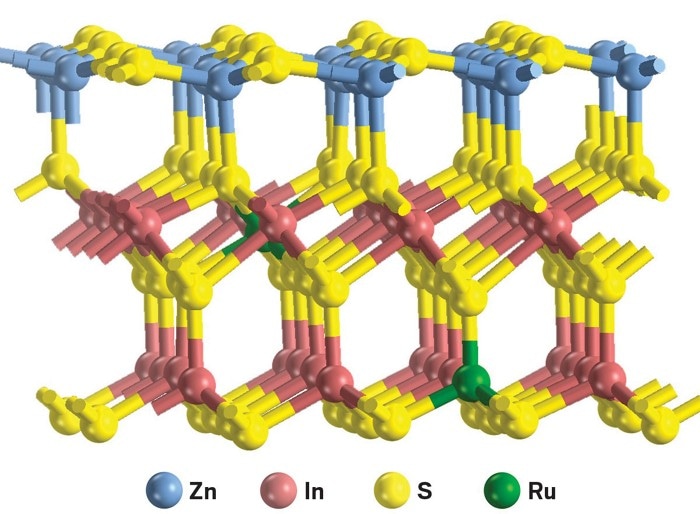
The researchers paid attention to two such compounds, namely, 2,5-dimethylfuran and 2-methylfuran, which are generally obtained from lignocellulose. The scientists investigated a series of metal-doped catalysts and discovered that during irradiation using visible light, Ru-doped ZnIn2S4 can readily combine with the furanics and produce dimers, trimers, and tetramers and release hydrogen.
Later, the scientists demonstrated that the products containing approximately 10–20 carbon atoms can potentially be used as precursors in producing diesel fuel. The researchers used the hydrogen released during the coupling reactions and a second catalyst to generate a mixture of linear and branched alkanes in the C12–C18 range characteristic of diesel with a yield of 76%.
According to Chao-Jun Li, a specialist in green chemistry and catalysis, from McGill University, this research describes a crucial innovation of using light energy to convert biomass into liquid fuels. Li stated that much of the earlier studies in this field were based on pyrolysis of biomass at high temperature, which produced a complex mixture of products.
One of the difficulties is to find efficient and careful ways of producing liquid fuels with long carbon chains. Le explained that this study is a significant improvement in that direction.
Li also stated that the scientists used LED light and had to supply more hydrogen for converting the precursors into fuel molecules. The study would attract even more interest if it is possible to use direct sunlight to produce the precursors and combine that process with solar-generated hydrogen.