Jul 25 2019
Researchers from the Institute of Solid State Physics, Hefei Institutes of Physical Science created an efficient transition metal electrocatalyst for enhancing oxygen evolution reaction. The study was reported in Small.
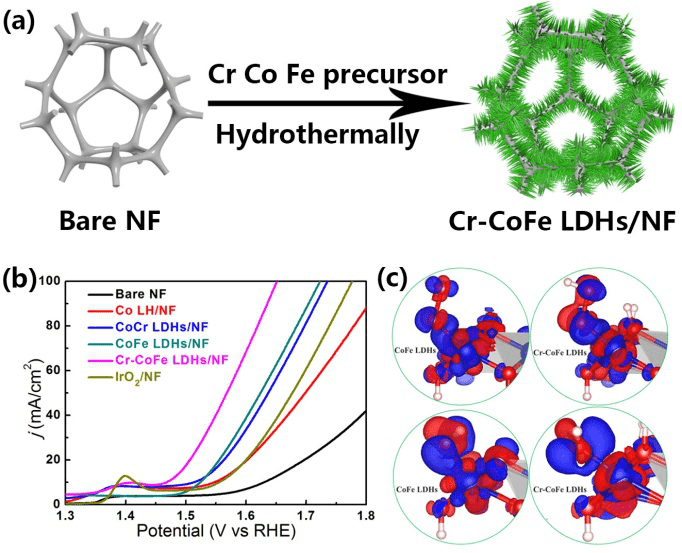 (a) Schematic preparation of Cr-CoFe LDHs/NF. (b) OER performance of Cr-CoFe LDHs/NF in 1 M KOH. (c) The charge density difference plots for OOH (upper panel) and OH (lower panel) on CoFe LDHs and Cr-CoFe LDHs with an isosurface value of 0.005 e.A-3. (Image credit: WEN Lulu)
(a) Schematic preparation of Cr-CoFe LDHs/NF. (b) OER performance of Cr-CoFe LDHs/NF in 1 M KOH. (c) The charge density difference plots for OOH (upper panel) and OH (lower panel) on CoFe LDHs and Cr-CoFe LDHs with an isosurface value of 0.005 e.A-3. (Image credit: WEN Lulu)
Due to the quick depletion of fossil fuels, the advancement of alternative clean energy sources is a pressing quest. One of the most potential technologies for the development of sustainable energy storage and conversion systems is electrochemical water splitting.
Sadly, the full water-splitting reaction is limited by the oxygen evolution reaction (OER) process, predominantly due to the slow kinetic process of the oxygen-related reactions.
Co-based layered double hydroxides (LDHs) exhibit a substantial OER activity and high binding energy of oxygen intermediates on the Co-active sites since the 3D band center rises from late transition metals to early ones in the periodic table.
The binding energies of the four oxygen intermediates stick to a linear relation, which obtains a theoretical limit of the overpotential of approximately 0.35 V. How to present effective approaches to infringe the theoretical boundary becomes important but challenging.
In this study, the group described an exclusive Cr-doped CoFe layered double hydroxides nanorod arrays supported on a nickel foam (Cr-CoFe LDHs/NF) electrode by a one-step hydrothermal strategy.
Following Cr doping, the Cr-CoFe LDHs/NF catalyst exhibited better durability and high activity for oxygen evolution reaction. Density functional theory (DFT) calculations revealed that Cr dopants as new active sites could enhance the electron-donation potential of the resultant Cr-CoFe LDHs.
The current results might offer insights into creating highly active OER catalysts for electrochemical water splitting.
This study was supported by National Science Fund for Distinguished Young Scholars, Natural Science Foundation of China, the Major Program of Development Foundation of Hefei Center for Physical Science and Technology.