Oct 15 2019
For the first time, scientists from the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University have demonstrated that an inexpensive catalyst can split water and produce hydrogen gas continuously for hours in the extreme environment of a commercial device.
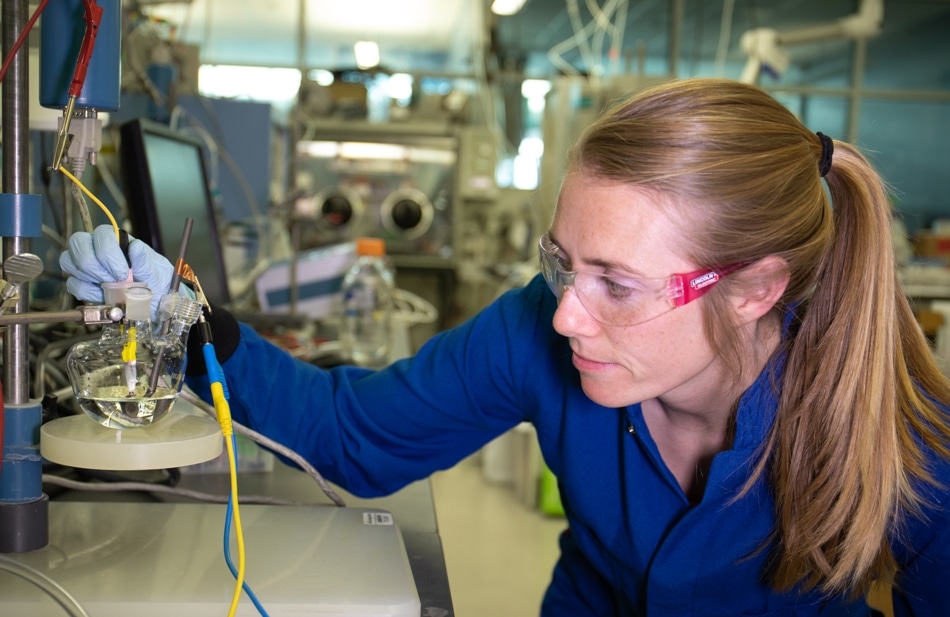 Stanford graduate student McKenzie Hubert with equipment used to test a cheap alternative to an expensive catalyst in the lab. A team led by Thomas Jaramillo, director of the SUNCAT center at SLAC and Stanford, went on to show for the first time that this cheap material could achieve high performance in a commercial electrolyzer. (Image credit: Jacqueline Orrell/SLAC National Accelerator Laboratory)
Stanford graduate student McKenzie Hubert with equipment used to test a cheap alternative to an expensive catalyst in the lab. A team led by Thomas Jaramillo, director of the SUNCAT center at SLAC and Stanford, went on to show for the first time that this cheap material could achieve high performance in a commercial electrolyzer. (Image credit: Jacqueline Orrell/SLAC National Accelerator Laboratory)
The electrolyzer technology based on a polymer electrolyte membrane (PEM) has the ability to produce hydrogen on a large scale, driven by renewable energy. However, it has been limited partly by the high cost of the precious metal catalysts, such as iridium and platinum, necessary to increase the efficiency of the chemical reactions.
The scientists reported recently in Nature Nanotechnology that this study opens the door that leads to an economical solution.
Hydrogen gas is a massively important industrial chemical for making fuel and fertilizer, among other things. It’s also a clean, high-energy-content molecule that can be used in fuel cells or to store energy generated by variable power sources like solar and wind. But most of the hydrogen produced today is made with fossil fuels, adding to the level of CO2 in the atmosphere. We need a cost-effective way to produce it with clean energy.
Thomas Jaramillo, Study Lead and Director, SUNCAT Center for Interface Science and Catalysis, Stanford University
From Expensive Metal to Cheap, Abundant Materials
Over the years, considerable work has been carried out to create substitutes for precious metal catalysts for PEM systems. A majority of them have been shown to function under laboratory conditions.
However, according to Jaramillo, this is the first study in which high performance in a commercial electrolyzer has been demonstrated. The device was made in a PEM electrolysis research site and factory in Connecticut for Nel Hydrogen, the oldest and largest manufacturer of electrolyzer equipment in the world.
Electrolysis functions quite similar to a battery in reverse: Instead of producing electricity, it uses electrical current to split water into oxygen and hydrogen. The reactions that produce oxygen and hydrogen gas occur on distinct electrodes using different precious metal catalysts.
Here, the Nel Hydrogen team swapped the platinum catalyst on the hydrogen-producing side with a catalyst containing cobalt phosphide nanoparticles deposited on carbon to create a fine black powder, which was synthesized by the scientists at SLAC and Stanford. Similar to other catalysts, it draws other chemicals together and stimulates them to react.
The cobalt phosphide catalyst worked very well for the entire period of the test, over 1700 hours—a sign that it may be sufficiently sturdy for daily use in reactions that can occur at higher pressures, temperatures, and current densities, as well as in very acidic conditions over prolonged periods of time, stated McKenzie Hubert, a graduate student in Jaramillo’s group.
Hubert guided the experiments along with Laurie King, a SUNCAT research engineer who has recently joined the faculty of Manchester Metropolitan University.
Our group has been studying this catalyst and related materials for a while and we took it from a fundamental lab-scale, experimental stage through testing it under industrial operating conditions, where you need to cover a much larger surface area with the catalyst and it has to function under much more challenging conditions.
McKenzie Hubert, Graduate Student, Stanford University
One of the study’s most significant elements was increasing the production of the cobalt phosphide catalyst while retaining its uniformity. This process required the synthesis of the starting material at the workbench, grinding with a mortar and pestle, furnace baking, and lastly converting the fine black powder into an ink that could be sprayed onto porous carbon paper sheets. The ensuing large-format electrodes were placed into the electrolyzer for the hydrogen production tests.
Producing Hydrogen Gas at Scale
While the development of the electrolyzer was sponsored by the Defense Department, which is keen on using the oxygen-producing side of electrolysis in submarines, Jaramillo stated that the study also supports the goals of DOE’s H2@Scale initiative, which unites DOE labs and industry together to achieve progress in the economical production, conveyance, storage, and use of hydrogen for several applications. The fundamental catalyst research was sponsored by the DOE Office of Science.
Working with Tom gave us an opportunity to see whether these catalysts could be stable for a long time and gave us a chance to see how their performance compared to that of platinum.
Katherine Ayers, Study Co-Author and Vice President for R&D, Nel Hydrogen
She continued, “The performance of the cobalt phosphide catalyst needs to get a little bit better, and its synthesis would need to be scaled up. But I was quite surprised at how stable these materials were. Even though their efficiency in generating hydrogen was lower than platinum’s, it was constant. A lot of things would degrade in that environment.”
Although the platinum catalyst signifies just about 8% of the total cost of producing hydrogen using PEM, the fact that the precious metal market is so unstable, with fluctuating prices, could restrain the development of the technology, stated Ayers.
Lowering and stabilizing that cost will become more and more important as other features of PEM electrolysis are enhanced to fulfill the growing demand for hydrogen in fuel cells and other applications.
SUNCAT is a joint collaboration between SLAC and the Stanford School of Engineering. This study was financially supported by a Small Business Innovation Research (SBIR) grant from the Department of Defense. Funding for the development of the fundamental catalyst at SUNCAT, which offered the platform for this study, was provided by the DOE Office of Science.