Nov 18 2019
A pair of pathways diverged in a chemical synthesis is taken up by a single molecule. At the University of Tokyo, chemists have explored how an ultrathin sheet or a spherical cage is formed by molecular building blocks.
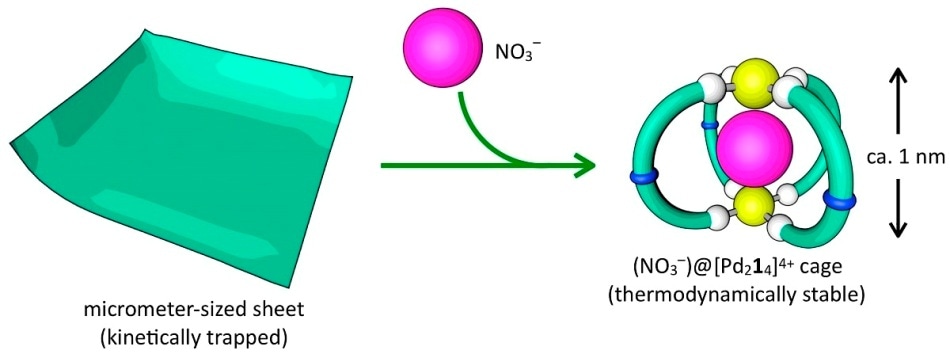 Chemists have mapped the reaction process where two types of molecules become either an ultrathin sheet (left) or a spherical cage (right), depending on the absence or presence of a third molecule, a negatively charged nitrate anion (red ball). If nitrate is added to the sheet, the molecules rearrange to form spherical cages. Image Credit: Shuichi Hiraoka, CC-BY-ND.
Chemists have mapped the reaction process where two types of molecules become either an ultrathin sheet (left) or a spherical cage (right), depending on the absence or presence of a third molecule, a negatively charged nitrate anion (red ball). If nitrate is added to the sheet, the molecules rearrange to form spherical cages. Image Credit: Shuichi Hiraoka, CC-BY-ND.
This sheet or cage exhibits some of the fundamental characteristics of a “smart” material that can react to its setting.
This molecule is interesting because it builds different structures depending on the conditions when it reaches the bifurcation point of its synthesis.
Shuichi Hiraoka, Professor, Department of Basic Science, The University of Tokyo
The research interests of Hiraoka are focused on how molecules are able to put themselves together, such as micelles or DNA in living cells. These molecules can be found in nature as well as in the cosmetics sector.
A “fork in the road” of the chemical synthesis pathway is the bifurcation point. The same precursor molecules in this pathway can join in two different ways to ultimately form different final structures. The precursors in the existing reaction take different paths based on the absence or presence of a third molecule.
Palladium metal atoms and an organic molecule called 1,4-bis(3-pyridyloxy)benzene are the precursor molecules. The organic molecule is made from three rings that effortlessly swing between a C-shape and an S-shape orientation.
The third molecule is a negatively charged anion molecule (either triflate or nitrate). The presence or absence of this molecule influences the type of path taken by the precursors.
If the anion is present, the organic molecule swings into the C-shape orientation, and four of those Cs sequentially join together into two O-rings and lock the anion in a spherical cage. A pair of palladium atoms joins the four Cs together at the bottom and top of the cage.
In the absence of anion, the organic molecule takes the S-shape orientation and joins together with other S-shaped molecules utilizing the palladium atoms as links. These molecules ultimately form flat sheets that have a thickness of approximately 4 nm and a diameter of up to 5 µm.
But when the anion is added to the completed sheet, the molecules tend to gradually reassemble themselves to form a cage.
The sheet is demonstrating some very primitive qualities of a so-called smart material—one that can sense and respond to its environment. This shift from the micrometer-sized sheets to the nanometer-sized cages is a very dramatic structural change.
Shuichi Hiraoka, Professor, Department of Basic Science, The University of Tokyo
The scientists believe that their analysis to interpret the underlying chemical characteristics of these molecules will pave the way for designing molecules that can assemble on their own and also individually reorganize based on environmental conditions.
Paths Depend on Thermodynamics and Kinetics
The formations of the cage and sheet are more chemically stable in numerous ways. The formation of the cage is more thermodynamically stable, which means it would need energy to shift out of that formation. When compared to the cage, the sheet is more kinetically stable, which means the molecules slowly change their position. The scientists are excited over the development of an artificial system that has the intricacies of these diverse stabilities.
“Complicated natural self-assembly reactions in living systems often have kinetic control,” Hiraoka explained.
Proteins found in living organisms are often kinetically trapped to remain in their healthy formations, although they would be more thermodynamically stable to combine into useless clumps.
In the new artificial system investigated by Hiraoka’s research group, after the cages are formed by the precursor molecules, the molecules remain in that final position since it is the lowest arrangement of thermodynamic energy.
The reaction in the early stage to form the cage is very fast, which tells us that the anion is acting as a kinetic template for the precursors to form the cage.
Shuichi Hiraoka, Professor, Department of Basic Science, The University of Tokyo
But the reaction to create the sheet progresses more gradually. According to the scientists, the molecules become kinetically trapped in the sheet formation in the absence of the anion to offer a template that draws them into the cage formation.
The scientists are planning to continue their exploration of how the self-assembly pathway is regulated and how to exploit the impact of the thermodynamic stability and the kinetic effect.