Feb 3 2020
A new non-flammable electrolyte applicable to potassium and potassium-ion batteries has been developed by Australian researchers. The electrolyte can be used in sophisticated energy-storage systems that are beyond lithium technology.
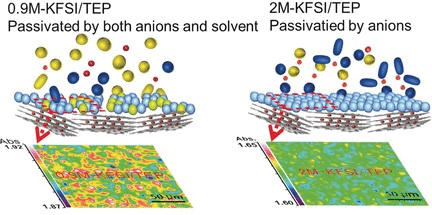 Image Credit: © Wiley-VCH, Angewandte Chemie.
Image Credit: © Wiley-VCH, Angewandte Chemie.
In the Angewandte Chemie journal, researchers describe that the latest electrolyte renders the potassium and potassium-ion batteries safer and also enables operation at low concentrations, a condition that is essential for commercial applications. The electrolyte is based on an organic phosphate.
Although lithium-ion technology continues to rule energy-storage applications, it has innate drawbacks, such as environmental problems, price, and the electrolyte’s flammability, to name a few.
Hence, in state-of-the-art technologies, researchers are substituting lithium ion with relatively cheaper and more abundant ions, for example, the potassium ion. But safety issues are also associated with both potassium and potassium-ion batteries, and non-flammable electrolytes for such batteries are not yet available.
Zaiping Guo, a materials scientist, and her research team from the University of Wollongong, Australia, have now identified a new solution. The scientists created an electrolyte, which was based on a flame-retardant material, and modified it for applications in potassium batteries.
Apart from offering non-flammability, the electrolyte could also be used in batteries at concentrations that are appropriate for commercial applications, the researchers wrote.
In this innovative electrolyte, the only solvent component is triethyl phosphate. This substance is referred to as a flame retardant. Although triethyl phosphate has been tested in lithium-ion batteries, only extreme concentrations of this substance offered sufficient stability for extended operations. But these concentrations are very high for large-scale applications.
The battery sector requires dilute electrolytes, which are not only cost-effective but also guarantee more improved performances. However, by utilizing potassium ions, the concentrations can potentially be decreased, reported the study authors.
The researchers mixed the phosphate solvent with potassium salt, which is commonly available, and eventually obtained a non-flammable electrolyte. This process also enabled steady cycling of the assembled battery concentrations ranging from 0.9 to 2 moles for each liter. These concentrations are appropriate for larger scales—for instance, in smart-grid applications.
According to the study authors, the formation of a stable and uniform interphase layer between the solid and the electrolyte is integral to that performance.
The researchers visualized this interphase layer, which guarantees electrode operability but only with the phosphate electrolyte.
Traditional carbonate-based electrolytes were incapable of forming this interphase layer. In addition, the study authors reported excellent cycling stability, while the traditional carbonate-based electrolyte decomposed under the same conditions.
Along with her research team, Guo has shown how a new kind of inorganic, phosphate-based electrolyte makes sophisticated potassium-ion batteries safer. The researchers also suggested that flame retardant-based electrolytes can be further developed and could be used for designing other types of non-flammable battery systems.