May 26 2020
The H+ proton contains a single ion of hydrogen, which is the lightest and smallest among all the chemical elements. Such protons occur in water naturally where a small proportion of H2O molecules gets split up instantaneously.
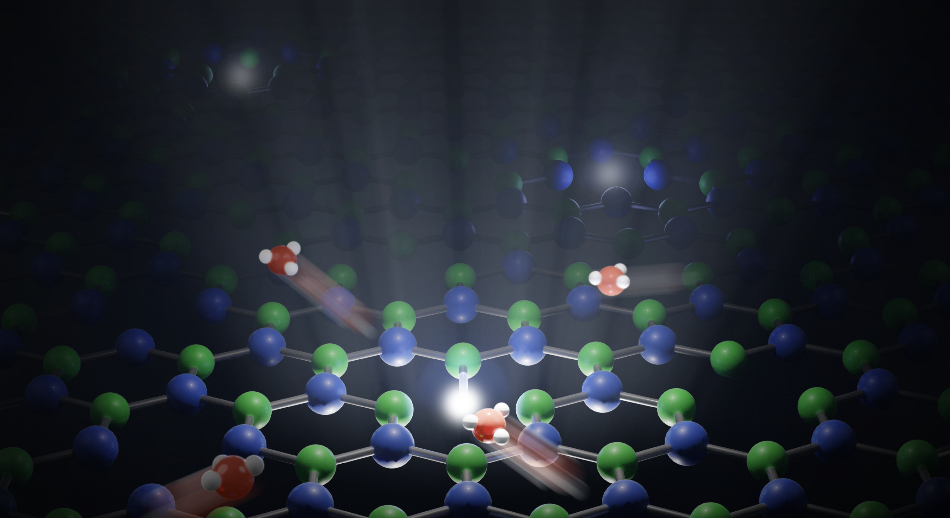
Image Credit: Ecole Polytechnique Federale de Lausanne (EPFL)
The amount of protons in a liquid decides if the solution is basic or acidic. Moreover, the protons are highly mobile, shifting via water by jumping from one water molecule to another.
Proton Transport at Water-Solid Interfaces
There has been a good understanding of the way this transport process functions in a body of water. However, the existence of a solid surface can drastically impact the behavior of protons, and at present, the researchers have very few tools to quantify such movements at water-solid interfaces.
In the latest study, Jean Comtet, a postdoctoral researcher at Ecole Polytechnique Federale de Lausanne (EPFL) School of Engineering (STI), has offered the first-ever insight into the behavior of protons when water comes into contact with a solid surface, diving down to the maximum scale of a single proton and single charge.
The study results were published in the Nature Nanotechnology journal and show that protons move along the interface between these two mediums. Scientists from the Department of Chemistry at the École Normale Supérieure (ENS) in Paris also contributed to the study by performing simulations.
Crystalline Defects
Comtet examined the interface between a boron nitride crystal (a very smooth material) and water.
The surface of the crystal can contain defects. We found that these imperfections act as markers, reemitting light when a proton binds to them.
Jean Comtet, Postdoctoral Researcher, School of Engineering, Ecole Polytechnique Federale de Lausanne
Comtet used a super-resolution microscope to view such fluorescence signals and quantify the location of the defects to within about 10 nm—a very high degree of accuracy. The more fascinating fact of the study is that it still offered a new understanding of how the crystalline defects are triggered.
We observed defects on the surface of the crystal lighting up one after another when they came into contact with water. We realized that this lighting pattern was produced by a single proton jumping from defect to defect, generating an identifiable pathway.
Jean Comtet, Postdoctoral Researcher, School of Engineering, Ecole Polytechnique Federale de Lausanne
A Major Experimental Breakthrough
One of the main findings of the study was that the protons move along the water-solid interface. According to Comtet, “The protons keep on moving, but hugging the surface of the solid. That’s why we see these kinds of patterns.”
Aleksandra Radenovic, professor at EPFL’s Laboratory of Nanoscale Biology (LBEN), stated that “This is a major experimental breakthrough that furthers our understanding of how charges in water interact with solid surfaces.”
Our observations, in this specific context, can easily be extrapolated to other materials and environments. These discoveries could have important implications in many other fields and disciplines, from understanding biological processes at the cell-membrane interface to designing more efficient filters and batteries.
Jean Comtet, Postdoctoral Researcher, School of Engineering, Ecole Polytechnique Federale de Lausanne
Journal Reference:
Comtet, J., et al. (2020) Direct observation of water-mediated single-proton transport between hBN surface defects. Nature Nanotechnology. doi.org/10.1038/s41565-020-0695-4.