Dec 14 2020
At the Institute of Industrial Science of The University of Tokyo, scientists have simulated the glass-forming potential of metallic mixtures by using molecular dynamics calculations.
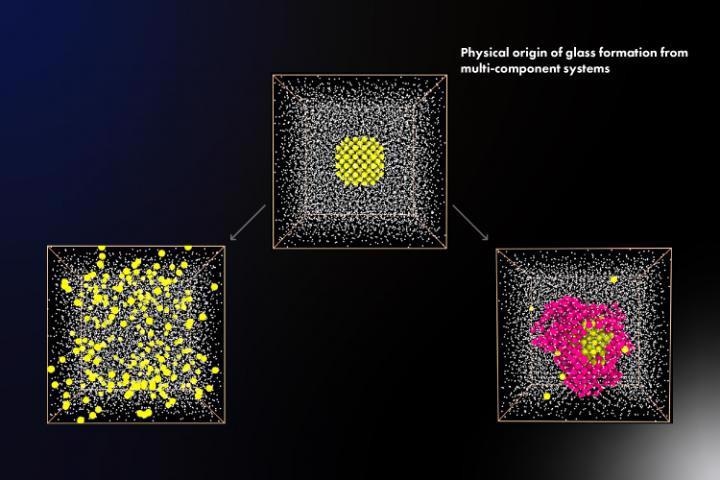 Scientists at The University of Tokyo use computer simulations to model the effects of elemental composition on the glass-forming ability of metallic mixtures, which may lead to tough, electroconductive glasses. Image Credit: Institute of Industrial Science, The University of Tokyo.
Scientists at The University of Tokyo use computer simulations to model the effects of elemental composition on the glass-forming ability of metallic mixtures, which may lead to tough, electroconductive glasses. Image Credit: Institute of Industrial Science, The University of Tokyo.
The team has demonstrated that even minuscule variations in composition could highly impact the probability that a material will take up a crystalline as against a glassy state when it is cooled. This study could pave the way for a universal theory of glass formation and more resilient, low-cost, electroconductive glass.
When people expect important guests for dinner, they might set the dinner table with high-cost 'crystal' glasses. However, for researchers, glass and crystal are actually two highly distinct states that might be assumed by a liquid upon cooling.
While a crystal has a defined three-dimensional lattice structure repeating indefinitely, glass is an amorphous solid without any long-range ordering. Existing theories of glass formation cannot precisely estimate the metallic mixtures that will 'vitrify' to form a glass and those that will crystallize.
While designing new recipes for electrically conductive materials that are mechanically tough, an improved, more in-depth understanding of glass formation would be helpful. Researchers from the University of Tokyo have now used computer simulations of three prototypical metallic systems to analyze the glass formation process.
We found that the ability for a multi-component system to form a crystal, as opposed to a glass, can be disrupted by slight modifications to the composition.
Yuan-Chao Hu, Study First Author, Institute of Industrial Science, University of Tokyo
In simple terms, the formation of glass is the result of a material avoiding crystallization upon cooling. Thus, the atoms are locked into a 'frozen' state before they arrange themselves into their energy-reducing pattern. The simulations demonstrated that the liquid-crystal interface energy is a critical factor that governs the rate of crystallization.
Moreover, the researchers discovered that variations in elemental composition could result in local atomic arrangements that hinder the crystallization process with arrangements not compatible with the usual form of the crystal.
These structures can particularly inhibit minuscule crystals from acting as 'seeds' nucleating the growth of ordered regions in the sample. Contrary to earlier explanations, the researchers identified that the difference in chemical potential between the crystal and liquid phases has only minimal effect on glass formation.
This work represents a significant advancement in our understanding of the fundamental physical mechanism of vitrification. The results of this project may also help glass manufacturers design new multi-component systems that have certain desired properties, such as resilience, toughness and electroconductivity.
Hajime Tanaka, Study Senior Author, Institute of Industrial Science, University of Tokyo
Journal Reference:
Hu, Y-C & Tanaka, H (2020) Physical origin of glass formation from multicomponent systems. Science Advances. doi.org/10.1126/sciadv.abd2928.