Feb 17 2021
The periodic table—an arrangement of the elements that helps identify and estimate the trends in their properties—is taught to all chemistry students. For instance, writers of science fiction occasionally explain life based on the silicon element, since it appears in the same column in the periodic table as carbon.
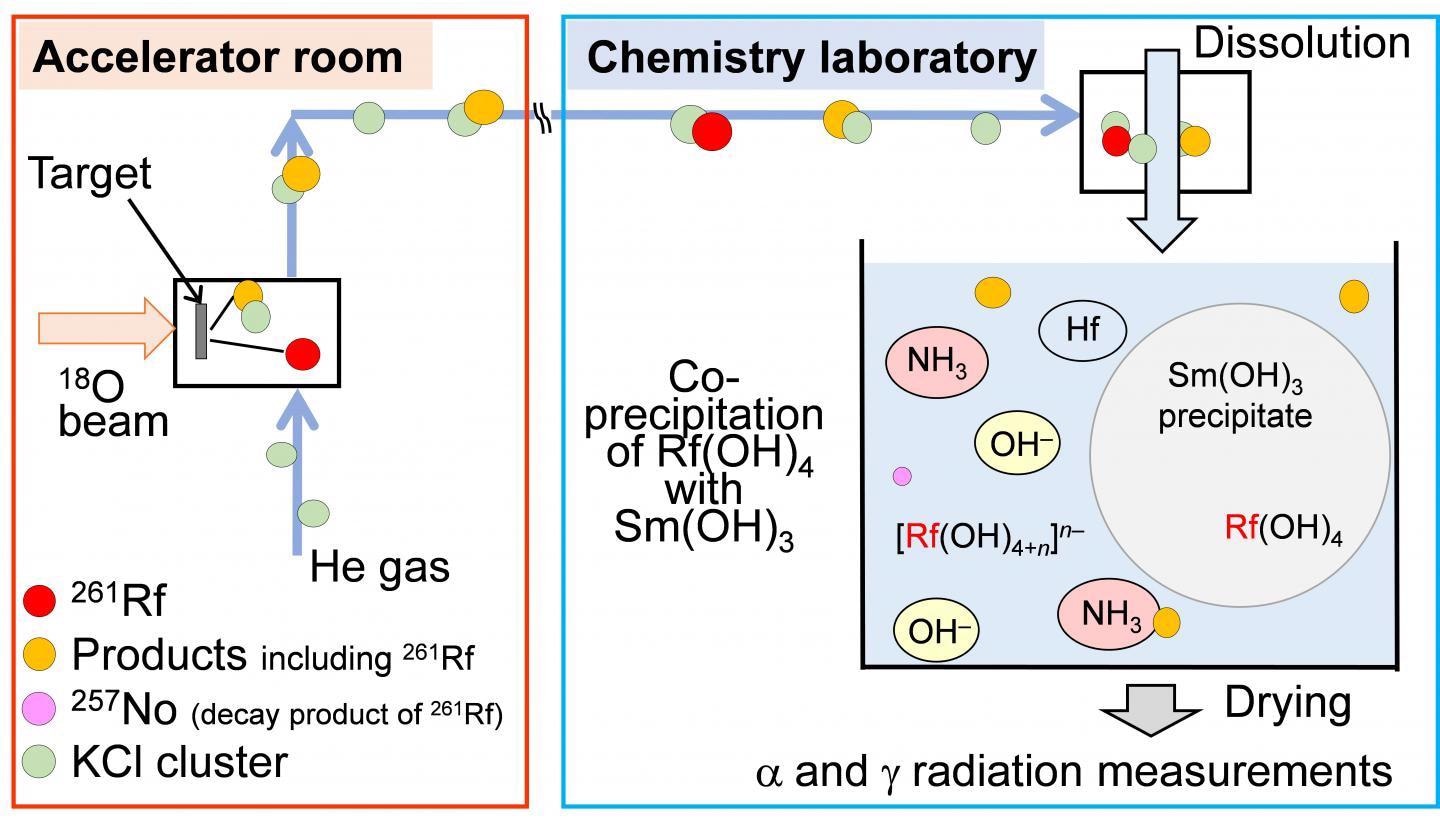 Schematic diagram of online co-precipitation experiment of 261Rf. Image Credit: Osaka University.
Schematic diagram of online co-precipitation experiment of 261Rf. Image Credit: Osaka University.
But there are some deviations from the expected trends of the periodic table. Tin and lead, for instance, are found in the same column in the periodic table and, hence, should have almost the same characteristics. But while lead-acid batteries are often used in cars, tin-acid batteries do not serve the same purpose.
Today, it is well known that the reason behind this aspect is that a large part of the energy in lead-acid batteries is attributed to relativistic chemistry; however; such chemistry was not known to the scientists who initially suggested the periodic table.
It is hard to study relativistic chemistry in superheavy elements. This is because these elements are usually created one at a time in nuclear fission reactions and they tend to decompose rapidly.
Nonetheless, the potential to analyze the chemistry of superheavy elements can perhaps reveal novel applications for superheavy elements and standard lighter elements, like gold and lead.
In a new study published in the Nature Chemistry journal, Osaka University researchers investigated the reactions between single atoms of superheavy rutherfordium metal and two groups of common bases. Experiments like these will allow scientists to use relativistic principles to better use the chemistry of several elements.
We prepared single atoms of rutherfordium at RIKEN accelerator research facility, and attempted to react these atoms with either hydroxide bases or amine bases. Radioactivity measurements indicated the end result.
Yoshitaka Kasamatsu, Study Lead Author, Osaka University
Such experiments will help scientists gain a better understanding of relativistic chemistry. For instance, rutherfordium can create precipitate compounds with hydroxide base at all concentrations of the base, but at high concentrations, it forms its homologs, hafnium and zirconium. This variation in reactivity could be attributed to relativistic chemistry.
If we had a way to produce a pure rutherfordium precipitate in larger quantities, we could move forward with proposing practical applications. In the meantime, our studies will help researchers systematically explore the chemistry of superheavy elements.
Atsushi Shinohara, Study Senior Author, Osaka University
Relativistic chemistry describes why bulk gold metal is not colored in silver, as one would anticipate based on the predictions of the periodic table. This chemistry also describes why mercury metal remains in a liquid state at room temperatures, in spite of the predictions of the periodic table.
There may be several unforeseen applications that emerge from learning about the chemistry of superheavy elements. Such discoveries will rely on recently reported procedures as well as ongoing fundamental analyses, like the one performed by the research team from Osaka University.
Journal Reference
Kasamatsu, Y., et al. (2021). Co-precipitation behaviour of single atoms of rutherfordium in basic solutions. Nature Chemistry. doi.org/10.1038/s41557-020-00634-6.