Apr 15 2021
The transfer of electrons between molecules forms the basis of biological energy flows, for example, in photosynthesis and respiration.
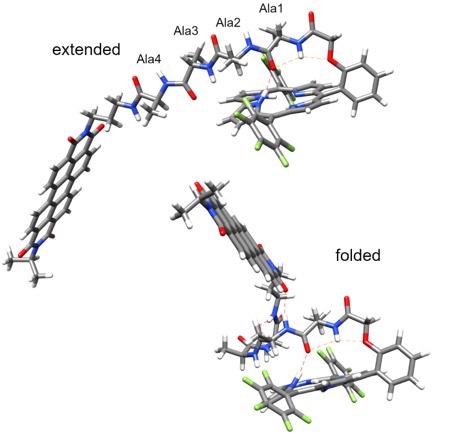 The extended peptide (top) does not mediate charge transfer (detectable charge transfer). The folded peptide (bottom) mediates picosecond charge transfer along the hydrogen bonds between the donor and the acceptor (the hydrogen bonds are indicated with thin red dotted lines). Image Credit: Valentine Vullev.
The extended peptide (top) does not mediate charge transfer (detectable charge transfer). The folded peptide (bottom) mediates picosecond charge transfer along the hydrogen bonds between the donor and the acceptor (the hydrogen bonds are indicated with thin red dotted lines). Image Credit: Valentine Vullev.
Although electron transfer is crucial for sustaining life, factors that determine its rate, particularly over long distances, are not clearly understood since the systems that mediate these ultrafast processes are highly complicated.
Gaining better insights into electron transfer rates would enable researchers to enhance photonic technologies, electronic devices, energy conversion and chemical transformations.
An international research group working under the guidance of the University of California, Riverside (UC Riverside), has noted picosecond charge transfer brought about by hydrogen bonds present in peptides. A picosecond is one-trillionth of 1 second.
Peptides are short-chain analogs of proteins, which are critically essential building blocks of living organisms, including chains of chemically linked amino acids. The breakthrough demonstrates the role of hydrogen bonds in electron transfer.
The study findings have been published in the Proceedings of the National Academy of Sciences.
Valentine Vullev, a professor of bioengineering at UC Riverside’s Marlan and Rosemary Bourns College of Engineering, together with Daniel Gryko from the Polish Academy of Sciences, and Harry Gray from the California Institute of Technology, headed a research group that found remarkably ultrafast electron transfer from a donor to an acceptor molecule linked with oligopeptide linkers expanding up to 20 covalent bonds.
Generally, electron transfer requires a microsecond, or one-millionth of a second, in peptides with very long through-bond distances. The team was fascinated to note picosecond electron transfer—a rate 1 million times quicker compared to what is earlier known for these systems.
It shouldn’t work, but it does. The picosecond charge transfer we observed contradicts structural biology, assuming the expected random distribution of structures of the flexible peptide chains.
Valentine Vullev, Professor of Bioengineering, Marlan and Rosemary Bourns College of Engineering, University of California, Riverside
The researchers selected donor and receptor molecules connected by short peptides they found actually assume clearly defined structures stabilized by hydrogen bonds.
Additional analysis showed that hydrogen bonds present inside each molecule brought the donor and acceptor next to each other in a scorpion-shaped molecular architecture, thereby allowing picosecond electron transfer.
This revolutionary design demonstrates short peptides can not only assume well-defined secondary conformations when templated by organic components but also provide a hydrogen-bonding network that can mediate electron transfer with unusually high efficiencies. Our work provides unprecedented paradigms for the design and development of charge-transfer pathways along flexible bridges, as well as insights into structural motifs for mediating electron transfer in proteins.
Valentine Vullev, Professor of Bioengineering, Marlan and Rosemary Bourns College of Engineering, University of California, Riverside
The study results could result in developments in energy storage as well as stimulate the advance of organic electronics that make use of conducting polymers rather than conducting minerals.
One of the most exciting and fulfilling aspects about working in our group is being at the forefront of such discoveries and observing these spectacular results.
John Clark, Study Co-Author and Doctoral Student in Vullev’s laboratory, University of California, Riverside
Photochemical measurements for the study were done by Clark.
The other authors of the study include Rafał Orłowski, Olga Staszewska-Krajewska, Hanna Jedrzejewska, and Agnieszka Szumna from the Polish Academy of Sciences; John A. Clark, James B. Derr, Eli M. Espinoza, and Maximilian F. Mayther at UC Riverside; and Jay R. Winkler from the California Institute of Technology.
Journal Reference:
Orłowski, R., et al. (2021) Role of intramolecular hydrogen bonds in promoting electron flow through amino acid and oligopeptide conjugates. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2026462118.