May 28 2021
Researchers from KAUST have illustrated an economical and dependable method to synthesize highly active single-atom catalysts by utilizing the exceptional metal-reducing potential of the iron-breathing bacterium Geobacter sulfurreducens.
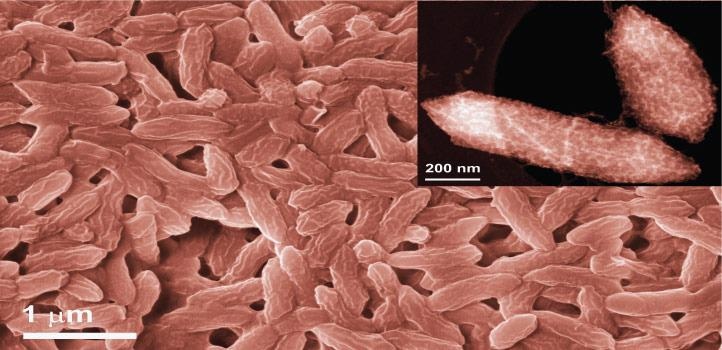 The research team exploited the iron-breathing bacterium Geobacter sulfurreducens to synthesize single-atom catalysts, which could be used for various energy-related applications. Image Credit: © 2021 KAUST.
The research team exploited the iron-breathing bacterium Geobacter sulfurreducens to synthesize single-atom catalysts, which could be used for various energy-related applications. Image Credit: © 2021 KAUST.
The invention, which could significantly enhance the cost and efficiency of hydrogen production from water, emphasizes the role that could be played by nature in the quest for new energy systems.
Several chemical reactions need a catalyst as a reactive surface where molecules or atoms gather with the correct amount of energy to trigger a chemical change. For instance, water could be separated into oxygen and hydrogen atoms by reacting on a pair of electrodes that are made of platinum and iridium oxide. But the reaction’s efficiency relies mostly on how several atoms can get involved.
In a nanoparticle catalyst, only 20 percent of the metal atoms might be available for catalysis. Single-atom catalysts, on the other hand, allow 100 percent atomic utilization and so are promising for various catalyst applications; however, conventional synthesis methods are expensive, involve high temperatures and only give low yields with poor atomic distribution.
Srikanth Pedireddy, University of Exeter
Pedireddy was formerly associated with KAUST.
Looking for an economical and more dependable method, Pedireddy, Pascal Saikaly, and their collaborators turned to nature. The anaerobic bacterium G. sulfurreducens is exceptional in that it “breathes” iron, not oxygen, and has the striking potential to conduct electrons from the cells’ inner side to outer side.
This bacterium has redox active proteins called c-type cytochromes that contain a heme complex—a central iron atom coordinated to four nitrogen atoms of a porphyrin ring. We envisaged that this heme site could be used to chemically reduce single atoms of catalytically active metals instead of iron.
Srikanth Pedireddy, University of Exeter
Following the verification of the development of single iron atoms at the cytochrome sites on the bacterial cells’ surface, the researchers immersed the bacteria in an iridium-containing solution, which generated a similar and very pleasing outcome.
Seeing single atoms on the surface of bacteria was a major challenge. With the high-resolution electron microscopy facilities at KAUST, we were able to visualize the atomically dispersed single atoms of metals on the bacteria surface.
Srikanth Pedireddy, University of Exeter
The researchers also discovered that the bacteria could be loaded with up to 1% of well-dispersed single-atom iridium, thereby providing a more reliable catalyst with hydrogen-evolving activity similar to the carbon or platinum standard at a fraction of the cost of other single-atom techniques.
“Our work could inspire the use of other efficient electroactive bacteria for synthesizing high-performing and low-cost electrocatalysts for various energy-related applications,” stated Saikaly.
Journal Reference:
Pedireddy, S., et al. (2021) Harnessing the Extracellular Electron Transfer Capability of Geobacter sulfurreducens for Ambient Synthesis of Stable Bifunctional Single-Atom Electrocatalyst for Water Splitting. Advanced Functional Materials. doi.org/10.1002/adfm.202010916.