Jun 15 2021
Recently, there has been a paradigm shift towards renewable energy sources to deal with concerns related to environmental degradation and declining fossil fuels. To reduce the global carbon footprints, a range of alternative green energy sources, like wind, solar, tidal, hydrothermal, etc., have been attracting a great deal of interest.
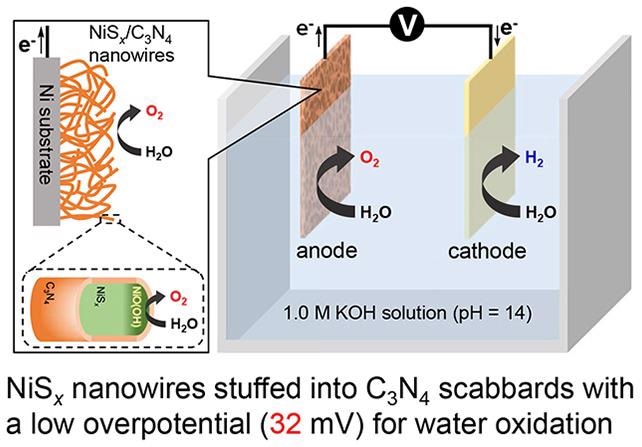 O₂ - NiSₓ/C₃N₄ anode: Schematic representation of the water dissociation process at low overpotential of about 32 mV using NiSₓ nanowires stuffed into C₃N₄ scabbard as anode for water oxidation. Image Credit: NIIGATA University.
O₂ - NiSₓ/C₃N₄ anode: Schematic representation of the water dissociation process at low overpotential of about 32 mV using NiSₓ nanowires stuffed into C₃N₄ scabbard as anode for water oxidation. Image Credit: NIIGATA University.
But a major challenge posed by these energy generation technologies is that they are both intermittent and are not available continuously.
We cannot use solar energy at night and wind energy when the wind is not blowing. But we can store the generated electricity in some other forms and utilize it whenever required. That is how water splitting bridges the gap and has emerged as a very promising energy storage technology.
Masayuki Yagi, Professor, Department of Materials Science and Technology, Faculty of Engineering/Graduate School of Science and Technology, Niigata University
Professor Yagi performs studies on energy storage materials and technology at the Department of Materials Science and Technology at Niigata University.
Water splitting is one of the viable energy storage solutions that may drive the world toward a hydrogen-fuelled economy.
The water dissociation process, also called artificial photosynthesis, conventionally uses electricity to split the water molecule via two half reactions in an electrochemical shell. The water oxidation takes place at the anode where breathable oxygen is discharged, and the hydrogen evolution reaction takes place at the cathode where hydrogen fuel is produced.
Even though water is a simple molecule containing three atoms, the dissociation process is quite intense and challenging.
The progress of the reaction is influenced by the initial energy, scientifically known as the overpotential. The overpotential or the initial energy needed to trigger the oxygen evolution at the anode and hydrogen evolution at the cathode is higher in the materials explored so far. This intense initial energy increases the overall cost of the reaction and thus negatively affects its commercial utilization.
This, in particular, is a major concern at the anode because the oxygen evolution reaction involves the transfer of four electrons and this requires a higher initial energy when compared to the reaction at the cathode.
Professor Yagi’s research team from Niigata University along with research collaborators from Yamagata University are exploring electrocatalytic water splitting, and to tackle the major shortcomings. They successfully developed an efficient water dissociation process using nickel-based nano-compounds as anodes. The study was published as a scientific article in the Energy & Environmental Science journal on May 20th, 2021.
Professor Yagi’s research team noted that the anode based on nickel sulfide nanowires has supported the reduction of initial energy that is needed for the oxygen evolution reaction.
We have fabricated the anode using a unique motif of nickel sulfide nanowires stuffed into carbon nitride scabbards. The carbon nitride scabbards prevent the core region of NiSx rods from transforming to their oxide, thereby protecting them from further degradation. On the surface of the nickel sulphide nanowires, a thin oxide film is formed due to the contact with the electrolyte solution, which facilitates the oxygen evolution reaction.
Masayuki Yagi, Professor, Department of Materials Science and Technology, Faculty of Engineering/Graduate School of Science and Technology, Niigata University
With the help of electrochemical measurements and advanced microscopy techniques, the researchers observed that the fabricated anode supports the reduction of initial energy, which speeds up the four-electron transfer process in the oxygen evolution reaction. The finding made by Professor Yagi's research team has huge potential in improving the long-term stability and performance of the electrochemical cell.
The study is a significant milestone towards enhancing the efficiency of the water splitting technology.
This result is a great breakthrough in the electrocatalytic water splitting system and could undoubtedly contribute to realize the de-carbonized human society in near future.
Masayuki Yagi, Professor, Department of Materials Science and Technology, Faculty of Engineering/Graduate School of Science and Technology, Niigata University
Journal Reference:
Zahran, Z. N., et al. (2021) Electrocatalytic water splitting with unprecedentedly low overpotentials by nickel sulfide nanowires stuffed into carbon nitride scabbards. Energy & Environmental Science. doi.org/10.1039/D1EE00509J.