An international team of researchers along with two Skoltech scientists has shown via an experiment that a long-standing interpretation for low energy efficiency in lithium-ion batteries is not valid.
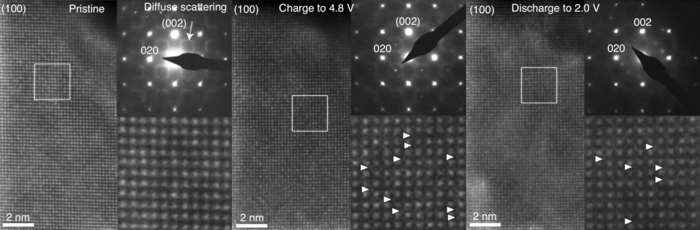 Transmission electron microscopy photos showing the atomic structure of the cathode at three different stages in the battery’s charge-discharge cycle. The white triangles indicate ions that are out of place or defects. Since the photo on the left shows the cathode in its pristine state, there are no defects. The ion migration apparent between the other two photos is vastly insufficient for explaining voltage hysteresis to the extent to which it is observed. (Image Credit: Biao Li et al./Nature Chemistry).
Transmission electron microscopy photos showing the atomic structure of the cathode at three different stages in the battery’s charge-discharge cycle. The white triangles indicate ions that are out of place or defects. Since the photo on the left shows the cathode in its pristine state, there are no defects. The ion migration apparent between the other two photos is vastly insufficient for explaining voltage hysteresis to the extent to which it is observed. (Image Credit: Biao Li et al./Nature Chemistry).
The phenomenon was explained by the team in terms of slow electron transfer between oxygen and transition metal atoms in the cathode, instead of the atoms themselves enduring migration. The study was published recently in the Nature Chemistry journal.
Currently, lithium-ion batteries used in electric vehicles and gadgets have around half the capacity their counterparts with lithium-enriched oxide cathodes. The downside with the latter technology is the low efficiency.
One has to use considerably more power to power the battery than it will provide in due course. Over time, and mainly for applications using up a lot of energy, this lost power certainly adds up, making those types of batteries not feasible for the market as of now.
To expose the capabilities of the batteries with lithium-enriched oxide cathodes, scientists have to comprehend the mechanism underlying their inefficiency and precisely where the lost energy goes.
The recent study offers experimental proof refuting the formerly held interpretation of the phenomenon — theoretically called voltage hysteresis — and provides a new theory to justify it.
As the lithium-ion battery powers up, lithium ions move between its two electrodes. When the ions travel toward the anode, they leave behind spaces in the cathode. In the other half of the cycle, lithium ions return as the energy gets used, for instance, to power a phone.
In the meantime, however, some of the transition metal atoms making up the cathode might have temporarily invaded the vacancies and then pulled back again, spending valuable energy on this jumping around. Or so the old theory of voltage hysteresis went.
Anatoly Morozov, Study Co-Author and PhD Student, Skoltech
The team tested this theory by using a transmission electron microscope at Skoltech’s Advanced Imaging Core Facility to track the atomic structure of a lithium-enriched battery cathode created from a material with the formula Li1.17Ti0.33Fe0.5O2 at various stages in the charge-discharge cycle of the battery.
However, they did not observe any noteworthy migration of titanium or iron atoms to lithium vacancies, indicating that some other process was tapping the power.
Our findings inspired the team to seek the origin of voltage hysteresis elsewhere. What gives rise to the phenomenon is not reversible cation migration but rather the reversible transfer of electrons between the atoms of oxygen and transition metals. As the battery gets charged, some of the electrons from iron are hijacked by the oxygen atoms. Later on, they go back. This reversible transfer consumes some of the energy.
Artem Abakumov, Professor and Head of the Center of Energy Science and Technology, Skoltech
Abakumov continued, “Understanding voltage hysteresis in terms of electron transfer might have immediate implications for mitigating this unwelcome effect to enable next-generation lithium-ion batteries with record-high energy density for powering electric cars and portable electronics.”
“To enable that next step, chemists could manipulate the electron transfer barriers by varying the covalency of the cation-anion bonding, guided by the periodic table and such concepts as ‘chemical softness’,” added Abakumov.
This demonstrates the power of advanced transmission electron microscopy for deciphering local structures of extreme complexity. It is really great that young researchers at Skoltech have direct and easy access to such sophisticated equipment as aberration-corrected electron microscopes, and opportunities for further training. This enables us to contribute to top-level battery research in collaboration with our international peers in both academia and the industry.
Anatoly Morozov, Study Co-Author and PhD Student, Skoltech
In addition to the two Skoltech chemists, the study included scientists from the University of Montpellier, Sorbonne, Collège de France, the University of Pau & Pays Adour, Technische Universität München, Paul Scherrer Institute, and Réseau sur le Stockage Electrochimique de l’Energie.
Journal Reference:
Li, B., et al. (2021) Correlating ligand-to-metal charge transfer with voltage hysteresis in a Li-rich rock-salt compound exhibiting anionic redox. Nature Chemistry. doi.org/10.1038/s41557-021-00775-2.