Producing clean energy is one thing - another challenge is to store this energy effectively. Research in the journal Energy Storage considers the use of tin disulfide in supercapacitors.
Study: A Review of Tin Disulfide (SnS2) Composite Electrode Materials for Supercapacitors. Image Credit: saran insawat/Shutterstock.com
Fossil fuel consumption is a major concern in the world today, and its demand is ever-increasing owing to the rapidly growing global economy. With the continuous upsurge in usage of fossil fuels globally, a couple of major challenges have arisen which have taken the world by storm.
The first is the rapid depletion of our fossil fuel reserves, and the second is the detrimental impact the use of fossil fuels has on the climate and the environment, giving rise to several global issues such as global warming and climate change, to name a few. The development of clean resources of energy, resources that do not jeopardize the environment, and supporting technologies, is therefore paramount.
Why Supercapacitors?
According to Setayeshmehr et al., portability is one of the most sought-after features in modern devices, which in turn requires inexpensive and small energy storage devices. Of the two most popular choices, batteries and supercapacitors, the latter is almost always preferred because it offers superior properties such as a longer life cycle and a much higher power density.
Supercapacitors consist of two electrodes, an electrolyte, and a separating material, that is anion permeable. The process of energy storage in supercapacitors can take place through one of two mechanisms: Faradaic transfer of charges via Faradaic processes, or the segregation of charges at the interface of electrodes and electrolyte in a Helmholtz double layer.
Supercapacitors can be classified into three main types, depending upon the method of energy storage: pseudo-capacitors, electrochemical double-layer capacitors (EDLCs), or a hybrid of the two. The performance of supercapacitors can be substantially increased by using a suitable material for the electrode to increase overall load storage.
The choice of materials for the electrode depends on several factors such as cost, conductivity, size of specific surface area and how unharmful they are to the environment. The importance of electrolytes cannot be overstated either.
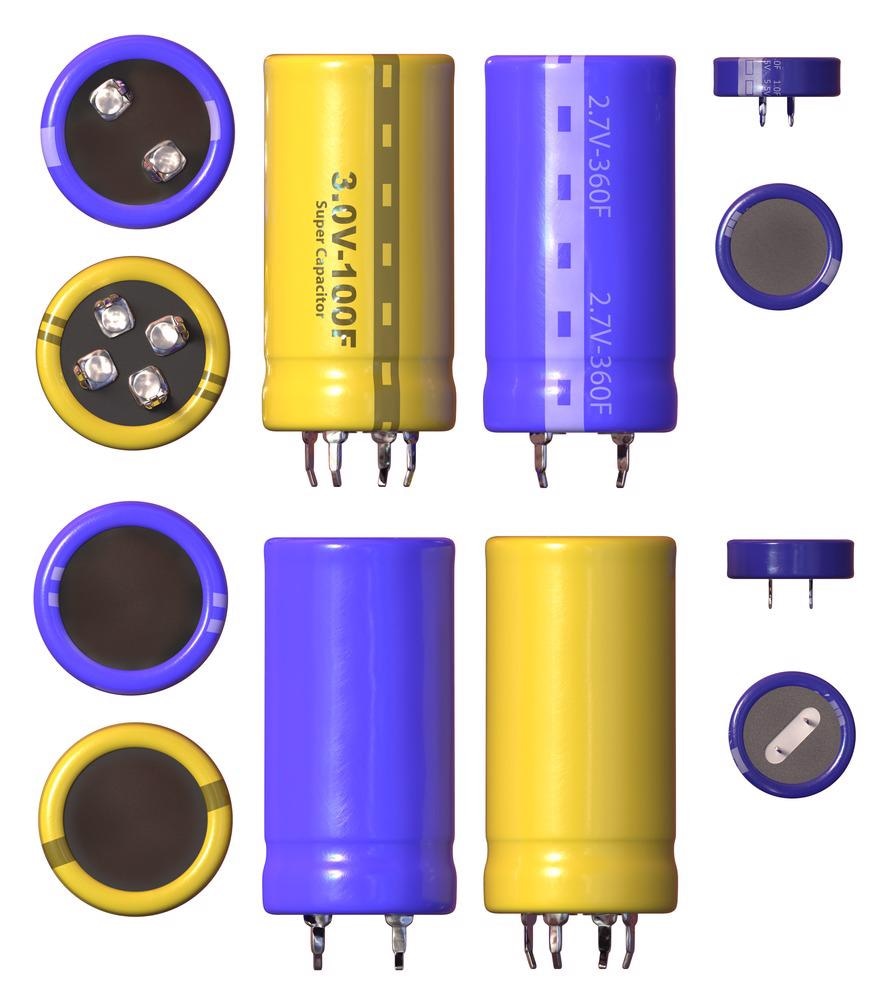
Image Credit: Peter Sobolev/Shutterstock.com
The properties of the electrolyte used, such as concentration, size, and type of ions, ion-solvent interactions as well as interactions between electrolyte and electrode materials, electrochemical stable potential window (ESPW), price, and chemical stability all have an impact on the life cycle, power and energy density, and capacitance of supercapacitors.
Choice of Electrode Material
Setayeshmehr et al. discuss how, on the grounds of recent studies, it has been revealed that the choice of electrode materials is not limited to metallic oxides, carbon-based materials, and polymers. A new choice has emerged, namely metallic sulfides.
The advantage of using these materials is that they offer higher conductive properties as compared to metallic oxides. An abundance of new research has revolved around discovering an electrode material that would offer increased power density and a longer cycle life as compared to the existing options, all at a low production cost.
A promising class of materials being studied greatly in this regard are two-dimensional transition metal dichalcogenides (TMDs), which sue elements like sulfur, tellurium, or selenium to bind with the transition metals. All this attention on TMDs is not unwarranted: their layered structures allow for higher power densities, much larger surface areas and higher conductivity.
Tin Disulfide
Metal chalcogenides, particularly tin (Sn) chalcogenides, have attracted a lot of attention recently. Of the various unique compounds of tin and sulfur (Sn-S), research efforts have been primarily focused on dual compounds including SnS, SnS2, Sn2S3, Sn3S4, and Sn4S5 due to the high potential upside they offer in terms of energy storage.
Among these select few compounds, tin disulfide (SnS2) is already seeing abundant use as the material of choice for capacitive electrodes owing to the large amounts in which it is available in the Earth’s crust, its non-toxic nature, and ease of stoichiometric control. Tin disulfide is the most promising electrode materials, when considering everything it offers, for the future of supercapacitors.
The composition and structure of this semi-conductor is quite interesting. Its crystals are made up of layers of cadmium iodide with S-Sn-S hexagonal units. Powerful ion-covalent bonds hold together the atoms, thus forming a plate-like morphology. Layers upon layers that are stacked over each other are connected by weak Van der Waals attractive forces.
These weak forces allow for the inclusion of Li or Na ions in the space between successive layers.
These unique structural and composition characteristics give tin disulfide electric bandgaps of 1.8-2.8 eV. In its current state, tin disulfide does have limited use in the world of supercapacitors due to its mediocre electric conductivity. However, if the electrical performance of this semi-conductor can be improved, it could revolutionize the world of supercapacitors.
Reference
Setayeshmehr, M, Haghighi, M, Mirabbaszadeh, K. (2021). A Review of Tin Disulfide (SnS2) Composite Electrode Materials for Supercapacitors. Energy Storage. https://onlinelibrary.wiley.com/doi/abs/10.1002/est2.295?__cf_chl_jschl_tk__=pmd_gIREt6.CpkOWw4WObshvz.wuYQjnqCQM5X0XWffWAbQ-1635256176-0-gqNtZGzNAiWjcnBszQ7R
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.