The latest research in Science Advances focused on a novel encapsulation strategy for perovskite cells by implementing lead-absorbing ionogel, which is quite effective in preventing leakage of lead and can withstand high-intensity impacts for a long time.

Study: Lead-adsorbing ionogel-based encapsulation for impact-resistant, stable, and lead-safe perovskite modules. Image Credit: Jozef Klopacka/Shutterstock.com
The latest research focuses on the vast usage of metal halides for low-cost power generation. However, perovskite containing water-soluble lead causes pollution of land, soil, and the underground water table.
Alternative Methods in the Past
Tin and double perovskite have been used to solve this problem, but tin-based PSCs have poor stability as Sn2+ is easily oxidized by Sn4+. A few other methods were also implemented, however they also had problems associated with them. Charge transport layers, such as alkoxy-poly tetra-ethylene glycol, were used.
However, these layers were not enough to absorb lead ions. Another famous method, to cover them with lead-absorbing material, failed as it was only able to slow down the rate of lead leakage not completely prevent it. Another major issue was the use of standard glass for PSC substrate which, due to inferior mechanical properties, shattered when damaged even a little bit.
The valid solution was ionogel-based encapsulation for perovskite modules, which have multiple functions of enhancing impact resistivity and reducing lead leakage, increasing the stability of perovskite modules.
Materials and Methods
The research mentioned that the materials used were anhydrous, 99% Sigma Aldrich, 95% Synthonix, AIBN (Sigma-Aldrich), and MBAA (Sigma-Aldrich).
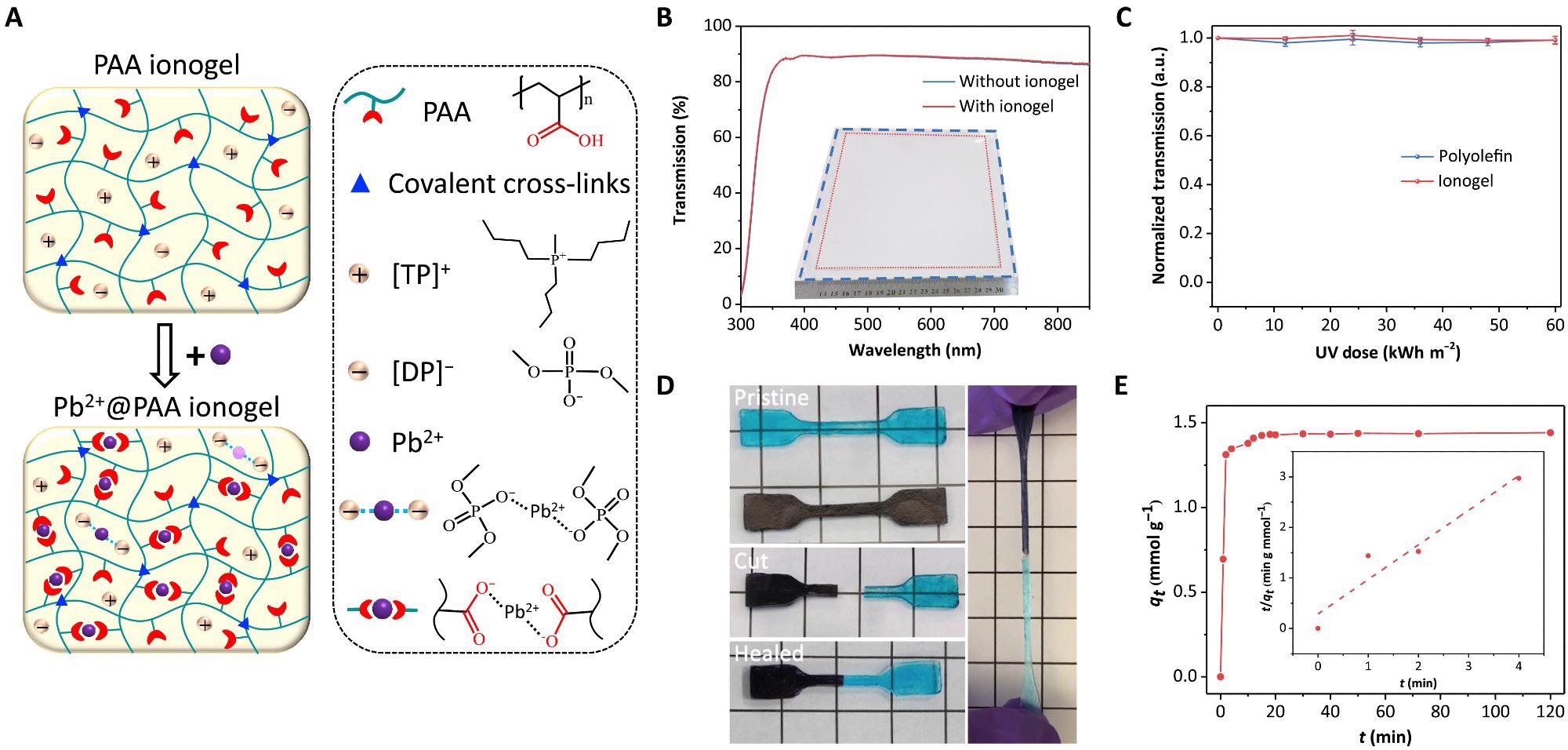
(A) Scheme of ionogel microstructure and lead adsorption mechanism. (B) Transmission of glass substrate with and without ionogel (thickness of 500 μm). Inset is the image of ionogel with an area of 15 cm by 15 cm (indicated by red dashed line) on a glass substrate (indicated by blue dashed line). (C) UV stability of ionogel and POE; error bar from three independent tests. (D) Pristine ionogel samples dyed with different colors (left top); images of cut samples (left middle) and healed at 50°C for 2 hours (left bottom); image of a stretched healed sample (right). The square (1 cm by 1 cm) in the image could be the scale reference. (E) Lead adsorption kinetics of ionogel. Inset is kinetics fitting curve from a pseudo–second-order model with an equation of tqt=1/(kqe)2 + tqe, where k is the rate constant and qe and qt are adsorbed lead ions at equilibrium and time t. Photo credit: Xun Xiao, University of North Carolina Chapel Hill.
By polymerizing AA monomers in TPDP, the ionogel was made in a single step. 0.001 g MBAA and 0.001 g AIBN were first dissolved in 0.4 g of AA monomer in a typical process; MBAA and AIBN were each 0.25 weight percent relative to AA. Hot-pressing under vacuum conditions was used to encapsulate the POE film. The tensile properties were measured using a tensile-compressive tester.
For the adsorption kinetics studies, 0.2 g of PAA ionogel was added to 50 ml of 8.6 104 M MAPbI3 aqueous solution and stirred continuously at 120 rpm. Different delay durations were used to extract specimens from the precursor solution.
Lead leakage Test
The effectiveness of encapsulation with PAA ionogel was found to prevent lead leakage due to the damage by solar minimodules. s. The MA0.7FA0.3PbI3 perovskite solar minimodules with an area of 52.7 ± 3.2 cm2 and the structure of ITO/PTAA/MA0.7FA0.3PbI3/C60/BCP/Cu was fabricated in one batch to avoid sample variation. The minimodules were subsequently encased with structures in POE or ionogel.
Minimodules with structure A fractured when they collided with the metal ball, however minimodules with constructions B and C remained intact with characteristic star-shaped fractures at the impact site. This emphasizes the importance of top-side encapsulation.
Lead leakage against Car Rolling
In addition to improved mechanical impact resistance, the robust ionogel in structure C was predicted to operate as a lead-adsorbing material, reducing lead leakage even more. To replicate a violent hailstorm, a highly damaging hail test was done with an impact density of 1 impact per square inch (i.e., 8 impacts for the modules employed here).
For samples A and B, cumulative lead leakage was 0.60 and 0.47 gm2, respectively. The color change was detected at the margin of the structure C module after 3 hours of soaking. Water had gotten into the perovskite layer, according to the findings. The quantity of accumulated lead leakage in the soaking water, on the other hand, did not exhibit a significant increase, with a final value of 7.8*105 gm2, which was four orders of magnitude lower than that in structure A.
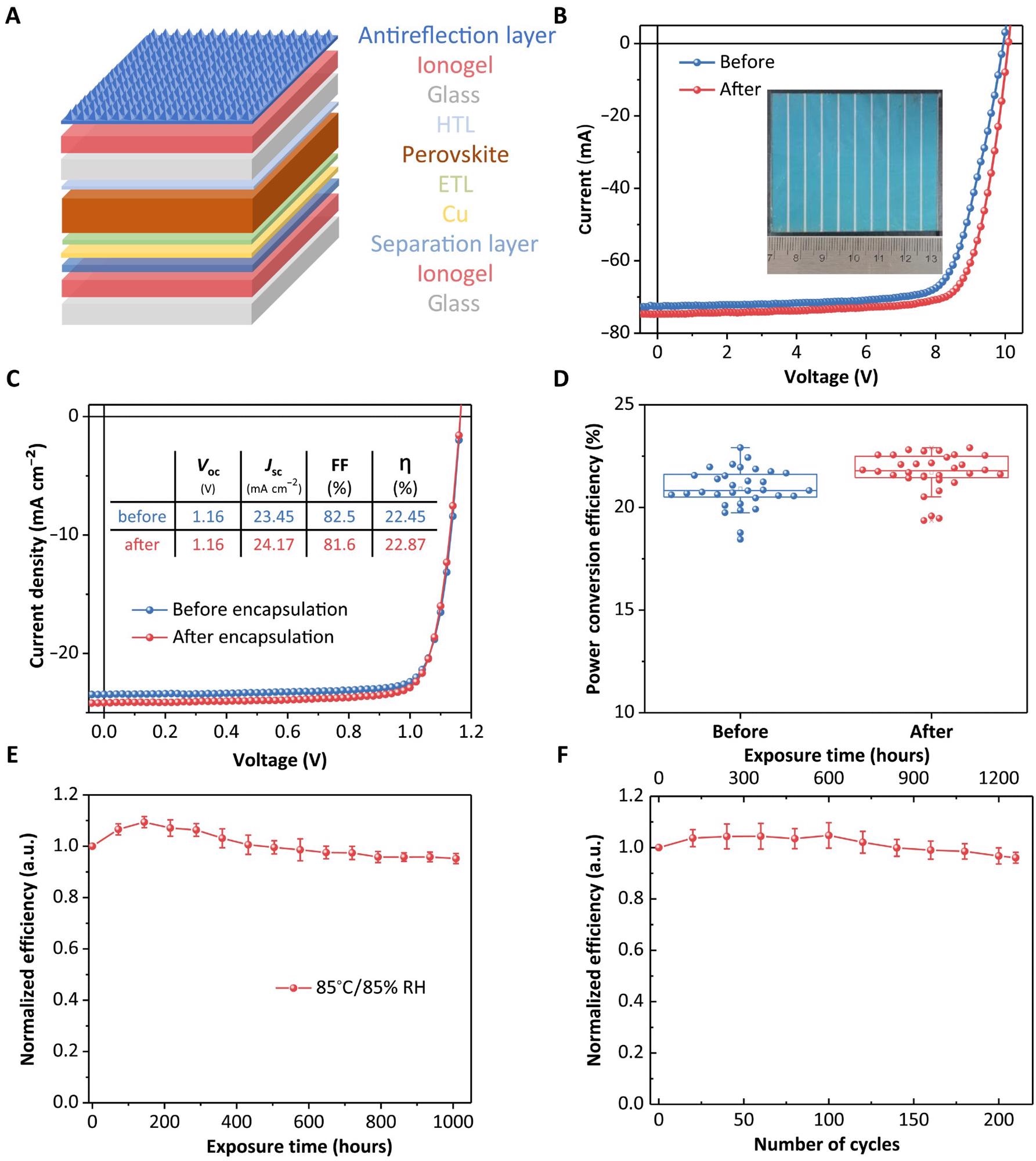
(A) Device structure. HTL, hole-transporting layer; ETL, electron-transporting layer. (B) I-V curves of perovskite module (area of 31.5 cm2) before and after ionogel encapsulation. Inset is the image of the encapsulated module. (C) J-V curves of typical PSCs (area of 0.08 mm2) before and after ionogel encapsulation. Inset is the chart of parameters comparison. (D) Statistic results of efficiency distribution of 32 PSCs before and after ionogel encapsulation. (E and F) Efficiency evolution of PSCs during DH (E) and thermal cycling (F) tests according to IEC 61215 standard. Error bars are from statistic results of five independent devices. RH, relative humidity. Photo credit: Xun Xiao, University of North Carolina Chapel Hill.
For the first 5 days of soaking, the encapsulated film of the ionogel-based structure C showed no significant color change and minimal lead leakage. The film began to change color after about 10 days of soaking, suggesting that water had infiltrated the perovskite layer and caused the breakdown.
When encapsulation with ionogel-based structure C is used instead of POE-based structure A, it predicts a nearly three-order-of-magnitude reduction in lead leakage.
The leakage of lead truly is a major issue and classical methods haven’t been able to cater to it successfully. However, the ionogel encapsulation passed all the standard tests and indicated superior mechanical and chemical properties. The tough, reliable, lead-absorbent material incorporated into perovskite solar module encapsulation fundamentally reduced the leakage of lead into the cells and also results in the enhancement of stability of the solar cells. The durable ionogel layer-based wrapping might remain untouched and operate as a filter layer to inhibit lead leakage even in harsh situations, such as being driven over by a car, indicating it is the best fit for this purpose.
References
Xiao, X., Wang, M., Chen, S., Zhang, Y., Gu, H., Deng, Y., . . . Huang, J. (2021). Lead-adsorbing ionogel-based encapsulation for impact-resistant, stable, and lead-safe perovskite modules. Science Advances. https://www.science.org/doi/10.1126/sciadv.abi8249
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.