The global reliance on fossil fuels has been a major factor in global warming, stemming from increased carbon dioxide (CO2) levels in the atmosphere. A new paper published in the journal Nature has showcased a further, potentially optimal, approach to the carbon-positive production of n-butanol.

Study: n-Butanol production by Rhodopseudomonas palustris TIE-1.Image Credit: Scharfsinn/Shutterstock.com
The urgent need to phase out fossil fuel consumption has prompted an increasing amount of research into the development and application of carbon-neutral biofuels – biofuels that do not result in net CO2 release when burned.
The biofuel n-butanol has thus far shown excellent promise and received significant attention from both industries and the scientific community. Its improved energy content, reduced volatility, and limited hydrophilicity – particularly when compared to biofuels based on ethanol – have garnered a great deal of interest.
One of the most useful characteristics of n-butanol is that it exhibits a number of similar properties to conventional gasoline, meaning that it can be used in traditional internal combustion engines to a degree, depending on their technology and material selections.
![n-Butanol biosynthesis pathway, cassette design, and major metabolisms used for n-butanol production in Rhodopseudomonas palustris TIE-1 (TIE-1). a n-butanol biosynthesis pathway involves five genes. The enzymes encoded by each gene and the reactions catalyzed by these enzymes are shown in dark blue. Two major byproducts (acetone and ethanol) are shown in dark red. NADH, nicotinamide adenine dinucleotide; NADPH, nicotinamide adenine dinucleotide phosphate. b Cassette design. The 3-gene cassette relies on phaA and phaB on the genome of TIE-1 for the first two steps of n-butanol synthesis. Here, only 3-genes (phaJ, ter, and adhE2) were introduced on a plasmid under a constitutive promoter PaphII. The 5-gene cassette has all five genes (phaA, phaB, phaJ, ter, and adhE2) on the plasmid under a constitutive promoter PaphII. c Photoheterotrophy: TIE-1 uses organic acids as carbon and electron source, light as an energy source, and ammonium (NH4+) or dinitrogen gas (N2) as a nitrogen source. d Photoautotrophy: TIE-1 uses carbon dioxide as carbon source, hydrogen (H2), ferrous iron [Fe(II)], or poised electrode as an electron source, light as an energy source, and NH4+ or N2 as a nitrogen source.???????](https://www.azom.com/images/news/ImageForNews_57249_16364587956058949.png)
n-Butanol biosynthesis pathway, cassette design, and major metabolisms used for n-butanol production in Rhodopseudomonas palustris TIE-1 (TIE-1). a n-butanol biosynthesis pathway involves five genes. The enzymes encoded by each gene and the reactions catalyzed by these enzymes are shown in dark blue. Two major byproducts (acetone and ethanol) are shown in dark red. NADH, nicotinamide adenine dinucleotide; NADPH, nicotinamide adenine dinucleotide phosphate. b Cassette design. The 3-gene cassette relies on phaA and phaB on the genome of TIE-1 for the first two steps of n-butanol synthesis. Here, only 3-genes (phaJ, ter, and adhE2) were introduced on a plasmid under a constitutive promoter PaphII. The 5-gene cassette has all five genes (phaA, phaB, phaJ, ter, and adhE2) on the plasmid under a constitutive promoter PaphII. c Photoheterotrophy: TIE-1 uses organic acids as carbon and electron source, light as an energy source, and ammonium (NH4+) or dinitrogen gas (N2) as a nitrogen source. d Photoautotrophy: TIE-1 uses carbon dioxide as carbon source, hydrogen (H2), ferrous iron [Fe(II)], or poised electrode as an electron source, light as an energy source, and NH4+ or N2 as a nitrogen source. Image Credit: Bai, W., Ranaivoarisoa, T.O., Singh, R. et al., Nature
A large proportion of n-butanol is synthesized using chemical processes that employ propylene or ethanol feedstocks, however—a method that is carbon-positive overall and negates the beneficial impact of n-butanol as a carbon-neutral fuel alternative.
A more sustainable means of n-butanol production does exist. Acetone–butanol–ethanol (ABE) fermentation via Clostridium species typically involves Clostridium acetobutylicum being introduced into a number of organisms, including Escherichia coli, Saccharomyces cerevisiae, Pseudomonas putida, and Bacillus subtilis. Many of these organisms are chemoheterotrophs, meaning that they must acquire energy from chemicals and consume other organisms to survive.
![High n-butanol production correlates to low acetone production amongst TIE-1 mutants. The concentration of acetone in mg/L when TIE-1 was cultured with ammonium (NH4+, red) or dinitrogen gas (N2, blue) and a acetate (photoheterotrophy) b 3-hydroxybutyrate (photoheterotrophy) c hydrogen (H2) (photoautotrophy) and d ferrous iron [Fe(II)] (photoautotrophy). CO2 was present in all conditions. Data are from n?=?3 of independent experiments. WT-3: wild type with 3-gene cassette; WT-5: wild type with 5-gene cassette; Nif-3: nitrogenase knockout with 3-gene cassette; Nif-5: nitrogenase knockout with 5-gene cassette; Gly-3: glycogen synthase knockout with 3-gene cassette; Gly-5: glycogen synthase knockout with 5-gene cassette; Phb-3: hydroxybutyrate polymerase knockout with 3-gene cassette, n.d. (non-detectable).???????](https://www.azom.com/images/news/ImageForNews_57249_16364588025885598.png)
High n-butanol production correlates to low acetone production amongst TIE-1 mutants. The concentration of acetone in mg/L when TIE-1 was cultured with ammonium (NH4+, red) or dinitrogen gas (N2, blue) and a acetate (photoheterotrophy) b 3-hydroxybutyrate (photoheterotrophy) c hydrogen (H2) (photoautotrophy) and d ferrous iron [Fe(II)] (photoautotrophy). CO2 was present in all conditions. Data are from n = 3 of independent experiments. WT-3: wild type with 3-gene cassette; WT-5: wild type with 5-gene cassette; Nif-3: nitrogenase knockout with 3-gene cassette; Nif-5: nitrogenase knockout with 5-gene cassette; Gly-3: glycogen synthase knockout with 3-gene cassette; Gly-5: glycogen synthase knockout with 5-gene cassette; Phb-3: hydroxybutyrate polymerase knockout with 3-gene cassette, n.d. (non-detectable). Image Credit: Bai, W., Ranaivoarisoa, T.O., Singh, R. et al., Nature
A new paper published in the prestigious Nature journal has presented a different approach to the carbon-positive production of n-butanol.
The study introduced the use of an anoxygenic photoautotroph Rhodopseudomonas palustris (TIE-1) to artificially synthesize n-butanol using a series of advanced metabolic engineering and hybrid bioelectrochemical platforms.
TIE-1 was found to be able to produce n-butanol using a range of different carbon sources - for example, organic acids and CO2, electron sources (H2, Fe(II), and a poised electrode) and nitrogen sources (NH4+ and N2).
A number of studies have found that oxygenic phototrophs such as cyanobacteria were able to produce biofuels using CO2, but the oxygen generated as part of oxygenic photosynthesis negatively impacted the efficiency of biofuel production.
Instead, the researchers aimed to specifically produce n-butanol from CO2, using the anoxygenic (non-oxygen evolving) photoautotroph, Rhodopseudomonas palustris TIE-1 (TIE-1).
The study specifically looked at a number of wild-type TIE-1 and mutants which did not exhibit electron-consuming (nitrogen-fixing) or acetyl-CoA-consuming (polyhydroxybutyrate and glycogen synthesis) pathways. Their goal was to evaluate which of these combinations of organism and pathway would produce the most optimal amounts of n-butanol.
A mutant that did not exhibit a nitrogen-fixing pathway was found to produce the highest levels of n-butanol.
One of the study’s most notable findings was that TIE-1 could produce n-butanol under photoelectroautotrophy. This means that TIE-1 is able to employ light to convert carbon dioxide into other compounds, potentially opening new pathways to more sustainable solar fuel production as solar energy could be used to efficiently induce n-butanol production.
In this context, n-butanol can be considered to be a ‘solar fuel’ due to the use of solar energy in its production.
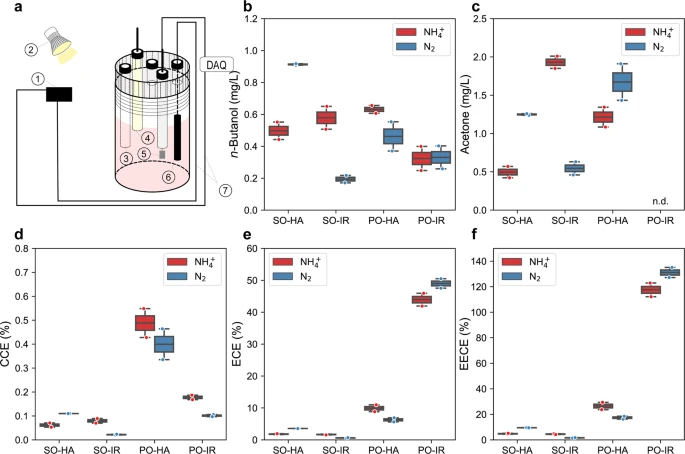
Three-electrodes configured sealed type bioelectrochemical cell (BEC), n-butanol production, acetone production, carbon conversion efficiency, electron conversion efficiency, and electrical energy conversion efficiency (EECE) towards n-butanol by the nitrogenase double mutant with the 5-gene cassette under photoelectroautotrophy. Under photoelectroautotrophic conditions, TIE-1 gains electrons from a poised electrode, using light as an energy source and carbon dioxide as a carbon source. For all the platforms, either ammonium (NH4+) or dinitrogen gas (N2) was supplied. a Schematic set up of BEC platform. platform set up: 1- electricity source 2-light source, 3- Purge inlet, 4- Reference electrode (Ag/AgCl in 3 M KCl), 5- Counter electrode (Pt foil, 5 cm2), 6- Working electrode (Graphite rod, 3.2 cm2), 7- electrical wire DAQ- Data acquisition); b n-butanol production; c acetone production; d carbon conversion efficiency (CCE) towards n-butanol; e electron conversion efficiency (ECE) towards n-butanol; f electrical energy conversion efficiency (EECE) towards n-butanol. PO potentiostat, IR infrared light, HA halogen light, SO solar panel. Data are from n = 2 of independent experiments.Image Credit: Bai, W., Ranaivoarisoa, T.O., Singh, R. et al., Nature
As pressure mounts to reduce global CO2 levels and make the transportation sector more sustainable, there is a growing need to explore alternative sources of fuel that are able to bridge the gap between current engine technology and new modes of transportation.
The ability to produce the popular biofuel n-butanol using entirely sustainable and carbon-positive means could open up new avenues to sustainable transport and other applications.
More so, the potential to employ a more sustainable fuel alternative in existing engine technology could make the transition to more environmentally transport much more appealing and accessible to consumers – a key consideration given the urgency of the need to reduce CO2 emissions and the increasingly stringent local and international targets around CO2 production.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Sources:
Bai, W., Ranaivoarisoa, T.O., Singh, R. et al. n-Butanol production by Rhodopseudomonas palustris TIE-1. Commun Biol 4, 1257 (2021). https://www.nature.com/articles/s42003-021-02781-z
International Energy Agency (IEA) Advanced Motor Fuels, (n.d.), Butanol, https://www.iea-amf.org/content/fuel_information/butanol/
US Department of Energy, (n.d.), Biobutanol, https://afdc.energy.gov/fuels/emerging_biobutanol.html