New research in the journal Green Chemical Engineering has presented an electrochemical process that reduces CO2 into usable formate.
Study: Nanoporous tin oxides for efficient electrochemical CO2 reduction to formate. Image Credit: PK Designs/Shutterstock.com
In recent years climate scientists and researchers have made it known that to preserve a livable climate greenhouse-gas emissions must be reduced to net-zero by 2050, at the latest. Modern industry has contributed to a significant increase in the concentration of carbon dioxide (CO2) in the atmosphere over the last 40 years – from around 339 parts per million in 1980 to 412 parts per million in 2020.
Reducing and converting CO2 into useful materials and chemicals remains a consistent challenge in trying to overcome the anthropogenic carbon cycle. However, a team of researchers at Tianjin University in China have presented an electrochemical process for the reduction reaction of CO2.
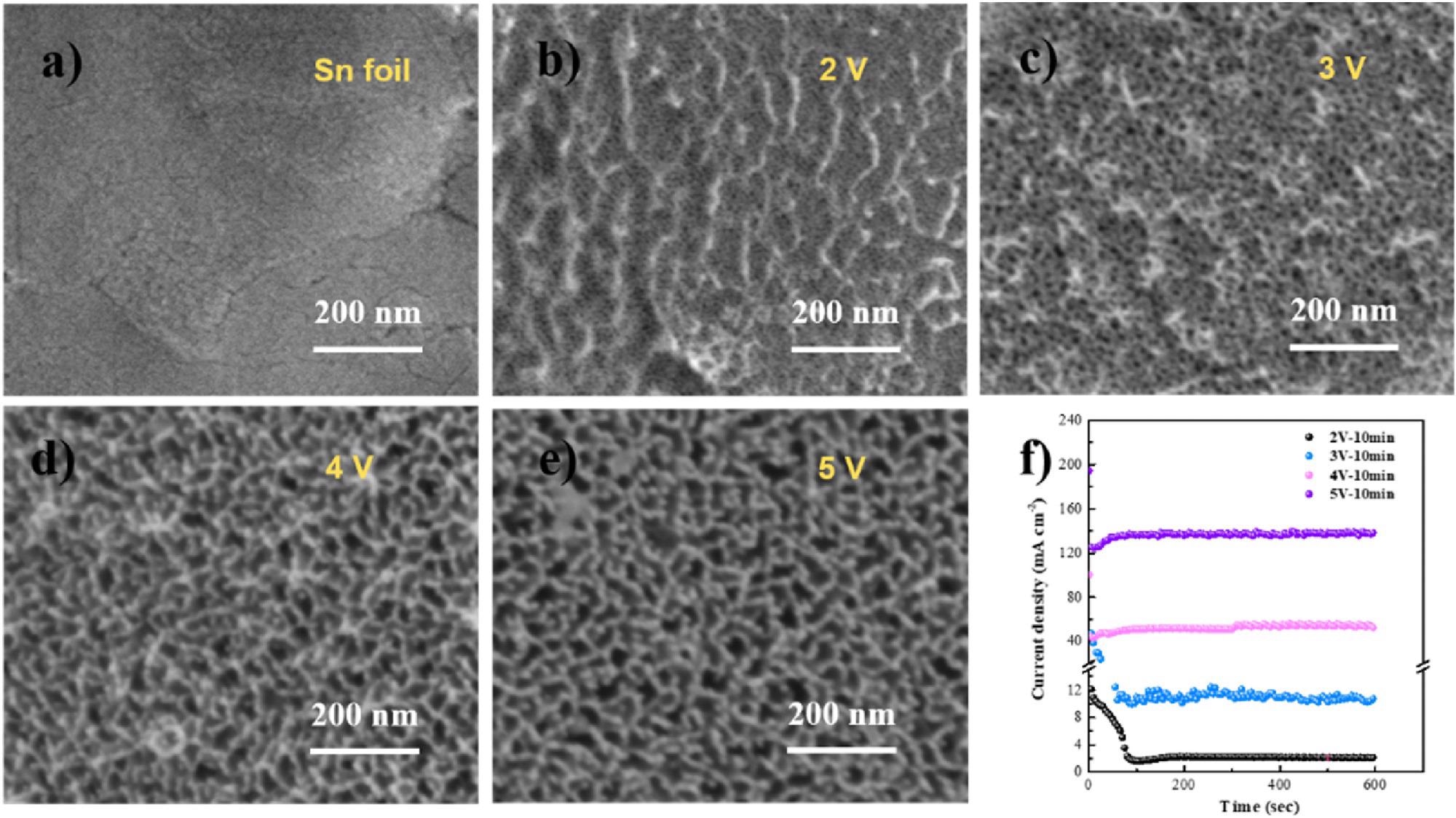 (a–e) SEM micrographs of Sn foil after anodization at various potentials in 1 M NaOH; (f) anodic oxidation i-t curves at various potentials in 1 M NaOH. Image Credit: Hai Liu et al., Green Chemical Engineering
(a–e) SEM micrographs of Sn foil after anodization at various potentials in 1 M NaOH; (f) anodic oxidation i-t curves at various potentials in 1 M NaOH. Image Credit: Hai Liu et al., Green Chemical Engineering
Using nanoporous tin oxides, the team demonstrated the potential for a scalable electrochemical anodic oxidation method. The ability to reduce CO2 to formate is appealing as it only requires two electrodes and can be performed at room temperature, with high atom efficiency.
Anodic Oxidation
The anodic oxidation method or electro-oxidation generates an oxide layer on a metallic substrate. Tin and its oxide have been previously identified as a suitable catalyst for electrochemical CO2 reduction reaction (CO2RR) and formate production due to a low environmental impact and at a relatively low cost.
“Nanoporous tin oxide catalysts were prepared by a simple one-step AO method, followed by scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS) to characterize the morphology and structure of the catalyst,” explained co-author of the study Professor Sheng Zang of the School of Chemical Engineering at Tianjin University.
Tin oxide-based nanomaterials can be vitally important in catalysis processes due to the presence of intrinsic acidic and redox characters. Additionally, the properties of tin oxide can be altered by inserting cation or anion species into its structure and interaction with other oxides.
Zang and his team believe that their results demonstrated a working method for the production of a nanoporous tin oxide catalyst for CO2RR. They also declared there was potential scope for industrial application of the method.
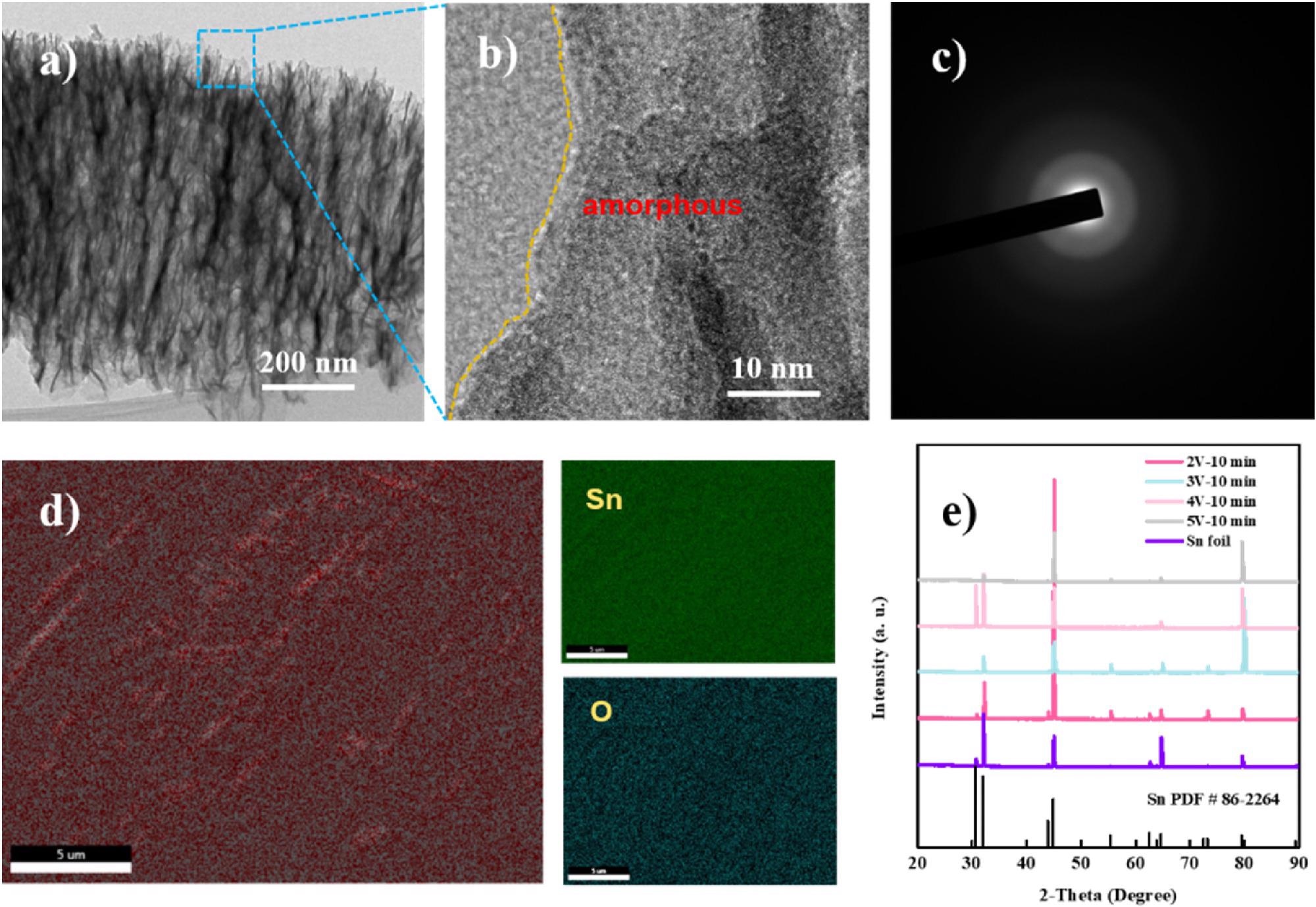
TEM (a), HR-TEM (b) and SAED (selected area electron diffraction) (c) images of porous SnO2; (d) SEM-element mapping images; (e) XRD pattern of the prepared Sn catalysts.Image Credit: Hai Liu et al., Green Chemical Engineering
CO2RR & Renewable Energy
Often, catalyst production for electrochemical methods can be time-consuming and costly, which restricts access to commercial scalability. Therefore, developing efficient cost-effective catalysts for selective electrochemical CO2RR to high-value products is crucial for the deployment of carbon utilization technologies.
In fact, making such progress could make a significant contribution towards carbon neutrality (Net-Zero), and CO2RR is deemed as one of the most promising approaches for the reduction of CO2 in the atmosphere and the production of alternative energy strategies.
“Among CO2 conversion technologies, electrocatalytic CO2 reduction reaction (CO2RR) has been considered as a most promising strategy to realize renewable energy storage and carbon neutralization,” Zang states.
As renewable electricity continues to decrease in price, CO2RR products, specifically those single carbon molecules such as carbon monoxide (CO) and formate, acquire a competitive price in contrast to traditional chemical engineering processes due to their industrially relevant selectivity.
There are still a number of challenges that necessitate further research to refine the CO2RR process to higher carbon products. One of these challenges is meeting the demand for actual industrial application, as CO2RR to formate demands a minimum of 200 mA·cm−2 current density and over 80% formate faradaic efficiency.
The China-based researchers were able to show that the nanoporous tin catalysts (SnO2) displayed superior formate faradaic efficiency compared with untreated tin foils: “In general, a 20–30% increases in formate faradaic efficiency was achieved after AO treatment,” Zang stated.
Additionally, the formate also demonstrated excellent properties which offered the team signs of encouragement: “Further flow cell test showed a formate partial current density of 285 mA·cm−2 with the selectivity of 96.4%, indicating a promising industrial application prospect,” explained Zang.
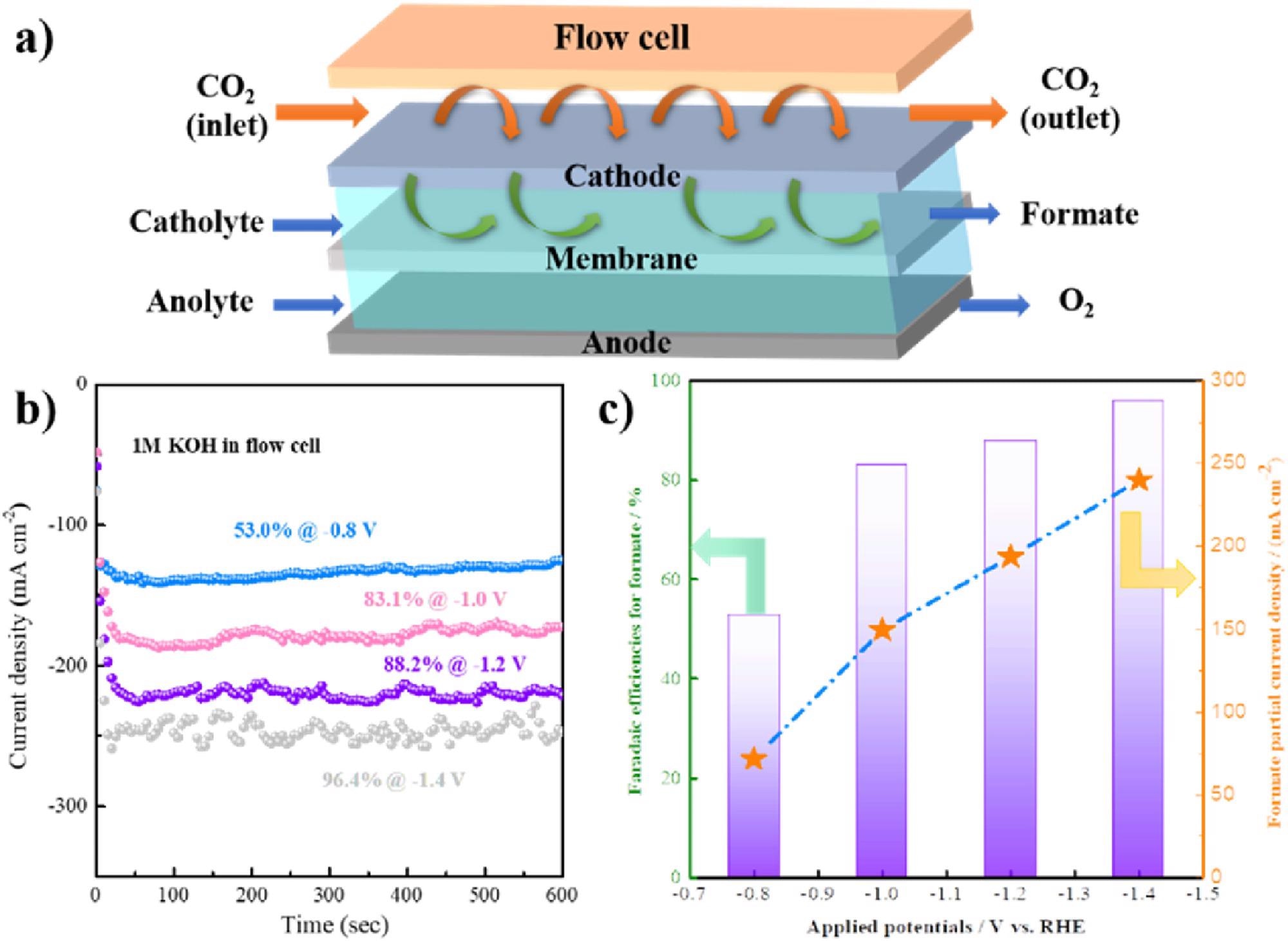
(a) Flow setup diagram; Potential-dependent i-t curves (b) and faradaic efficiencies and partial current density (c) of formate at various potentials of porous SnO2. Image Credit: Hai Liu et al., Green Chemical Engineering
If CO2RR and the simple, cost-effective production of catalysts can be realized in the near future it could open up the door for a revolution in green-energy storage and supply. The electrochemical conversion of CO2 to fuels and even feedstocks is a sophisticated solution to ending the carbon cycle when coupled with other renewable energy sources.
Formic acid and its salt (formate) are considered one of the most valuable CO2RR products due to their wide application potential in various fields. From being widely used in feedstock, and leather tanning processes to use in fuel cells and electronics, it’s clear to see how developing reduction and conversion processes could change the way we think about CO2.
References:
Hai Liu, Baiyu Miao, Hongyuan Chuai, Xiaoyi Chen, Sheng Zhang, Xinbin Ma, Nanoporous tin oxides for efficient electrochemical CO2 reduction to formate, Green Chemical Engineering, 2021. https://www.sciencedirect.com/science/article/pii/S2666952821000728?via%3Dihub
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.