Researchers from China recently developed a strategy to reuse waste graphite anodes of spent lithium-ion batteries. They transformed the recovered graphite into a new cathode material for dual-ion batteries through a two-step treatment.
The recycled graphite shows improved resistance to degradation and enhanced cycling performance. This simple approach further adds more sustainability to lithium-ion-based energy solutions. This study is available in the journal eScience.

Study: Advanced cathode for dual-ion batteries: Waste-to-wealth reuse of spent graphite from lithium-ion batteries. Image Credit: Lightboxx/Shutterstock.com
Reuse of Graphite from Anode Material: A Sustainable Approach
With an increase in the demand for lithium-ion batteries (LiBs) and scarcity of electrode materials, the energy sector is experiencing a push for more sustainable approaches. Owing to high energy density, long cycling life, and excellent portability, LiB will remain the highly demanded option for the next several years.
Currently, the commercial cathode materials in LiBs consist of various oxides or phosphates of lithium (Li), cobalt (Co), nickel (Ni), manganese (Mn), and iron (Fe), whereas common anode materials are carbon-based. The reuse of the spent carbon materials in cathodes can resolve the economic, environmental, and ethical dilemma.
The most common carbon materials in the anodes of LiBs are graphite, soft carbon, hard carbon, carbon nanotubes.
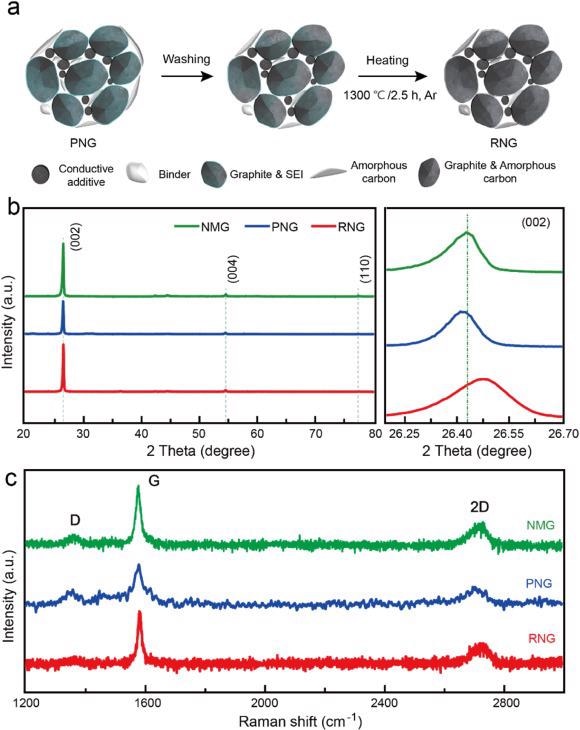
(a) Schematic diagram of the synthesis process for RNG; (b) XRD patterns; (c) Raman spectra of NMG, PNG, and RNG. Image Credit: Yang, J et al., eScience
Graphite dominates the LIB anode market because of its special layered structure that facilitates relatively stable insertion and extraction of Li+ ions. Also, it has good stability, high conductivity (~0.4 eV bandgap), lower lithium insertion potential (0.01-0.2 V), and higher theoretical capacity (372 mAh g–1) than other carbon materials.
Traditionally, pyrometallurgy is the primary method to recover graphite from anodes. In pyrometallurgy, graphite is incinerated together with cathodes, which is energy inefficient and releases plenty of greenhouse gases.
Synergy Between Dual-ion Batteries and Graphite
Dual-ion batteries (DIBs), have garnered a lot of attention due to the advantage of high output voltage and high energy density. In DIBs, cations and anions participate in the electrochemical reactions of the battery simultaneously; the anions undergo intercalation reactions just like alkali metal ions.
At the same time, carbon materials have been widely studied as electrode materials that permit the intercalation of various cations and anions. Among them, graphite materials have experienced the most development as cathode materials for DIBs. Thus, a combination of low-cost carbon materials and spent alkaline metal-ion batteries for cathodes in DIBs is efficient and sustainable.
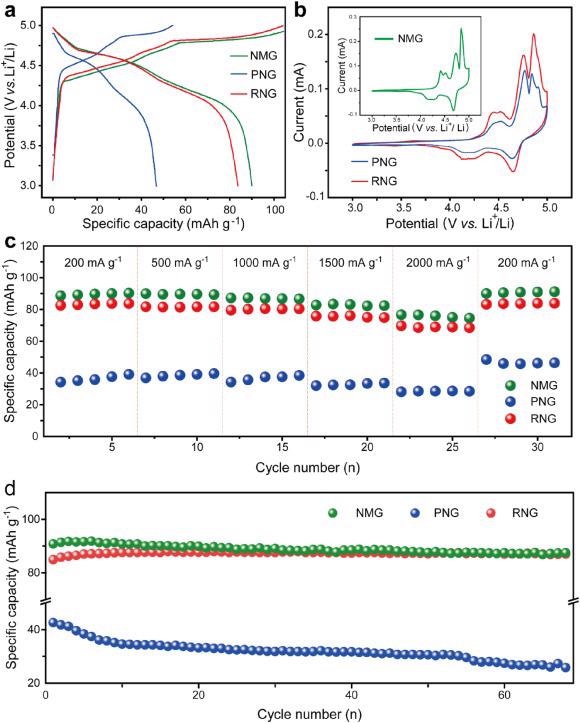
(a) GCD curves at 200 mA g−1; (b) CV curves; (c) rate performance and (d) cycling performance of NMG, PNG, and RNG. Image Credit: Yang, J et al., eScience
About the Study
The researchers procured recycled negative graphite (RNG) from anodes of LiBs and transformed it into cathodes of DiBs through a facile two-step process.
To obtain RNG, primitive negative graphite (PNG) powder recovered from the negative electrodes of waste LIBs is mixed with pure ethanol, and the obtained samples are heated at 1300 ℃ for 2.5 hours in an argon atmosphere. Finally, several experiments were carried out to measure the performance of the prepared sample and compared with that of PNG and natural modified graphite (NMG).
Observations
From X-ray diffraction (XRD), it is evident that the (002) peak of RNG shifts to the right in comparison with NMG, whereas that of PNG shifts to the left, indicating the layer spacing of RNG decreases while that of PNG increases.
After the heat treatment, the graphite layer spacing decreases whereas the packing density and degree of order increases in the graphite. Also, it shows that the degree of graphitization of RNG is 87.74%, which is higher than that of PNG and NMG.
This recovery method reinstates the morphology and crystal structure of end-of-life graphite. The distorted layer structure of graphite, after long cycling, is restored with appropriate layer spacing for anion intercalation through the coating of pyrolytic carbon. Additionally, the pyrolysis of the solid electrolyte interphase (SEI) component forms an amorphous carbon layer that prevents degradation of the electrode and enhances its cycling performance.
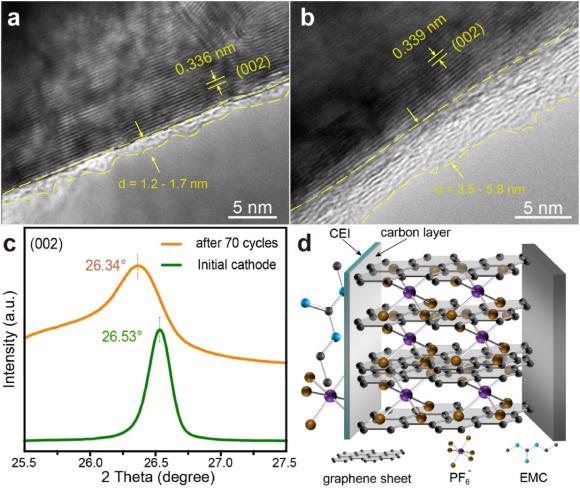
HRTEM images of (a) initial RNG and (b) RNG after 70 cycles. (c) XRD patterns of initial RNG and RNG after 70 cycles. (d) Proposed mechanism for GICs intercalated with PF6− in RNG. Image Credit: Yang, J et al., eScience
What is the Performance of the RNG?
The researchers developed a facile recycling method through a simple two-step process. The end-of-life anode graphite from LIBs is directly converted to RNG for use as a cathode in DIBs. The recycling method reinstates the crystal structure and morphology of RNG and restores its regular layer structure with preferable layer spacing. The RNG demonstrates a high reversible capacity of 87 mAh g–1 at 200 mA g–1, which was equivalent to that of commercial new graphite.
The RNG can be successfully used in Li-DIBs, Na-DIBs, and K-DIBs. This facile recycling method turns end-of-life anode graphite into a cathode material with promising potential and superior electrochemical performance, along with a sustainable energy approach.
Reference
Yang, J., Zhao, X., Li, W., Liang, H., Gu, Z., Liu, Y., Du, M., Wu, X., Advanced cathode for dual-ion batteries: Waste-to-wealth reuse of spent graphite from lithium-ion batteries, eScience, 2021, ISSN 2667-1417, https://www.sciencedirect.com/science/article/pii/S2667141721000227
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.