A new breakthrough was considered in the journal Materials of synthesized biphasic calcium phosphate ceramics (CaPs) to make tricalcium phosphate (-TCP) and hydroxyapatite (HAp) using coral.

Study: Biomimetic Ceramic Composite: Characterization, Cell Response, and In Vivo Biocompatibility. Image Credit: Sam0704/Shutterstock.com
Using a solid-state synthesis followed by heat processing at 1100 °C for 1 h to 7 days, SEM, X-ray diffractometry, Fourier transform infrared spectroscopy, and Raman spectroscopy was used to evaluate the as-prepared coral and coral-derived biphasic CaPs samples. In vitro cytotoxicity testing on mouse fibroblast cells was used to determine the cell response of the biphasic CaPs.
Coral Exoskeletons as a Calcium Crystals Source
Coral exoskeletons feature a unique linked porous design with tubular spaces ranging in size, comparable to normal teeth and bones, and have as such piqued the interest of orthodontic and maxillofacial surgeons. The existence of macropores larger in diameter promotes nutrient transport, osteogenesis, and tissue regeneration.
Coral exoskeletons are calcium carbonate crystals such as aragonite or calcium. Their disintegration rate as a skeletal growth rate is too quick to allow adequate bone ingrowth, limiting clinical applications.
Method to Create Coral Derived HAp
The recommended method for producing coral-derived HAp is hydrothermal conversions. However, this synthetic technique is unsuitable for large-scale production since it is slow, requires intricate pH changes, and has a small -TCP.
Using varied high-temperature heats and periods, a homogenous combination of calcium and phosphate precursors can be combined in water at room temperature to synthesize a biphasic CP with a regulated ratio of HAp to -TCP via the solid-state reaction pathway. Extremely crystalline and biphasic CaPs were produced with great repeatability and low operating cost.
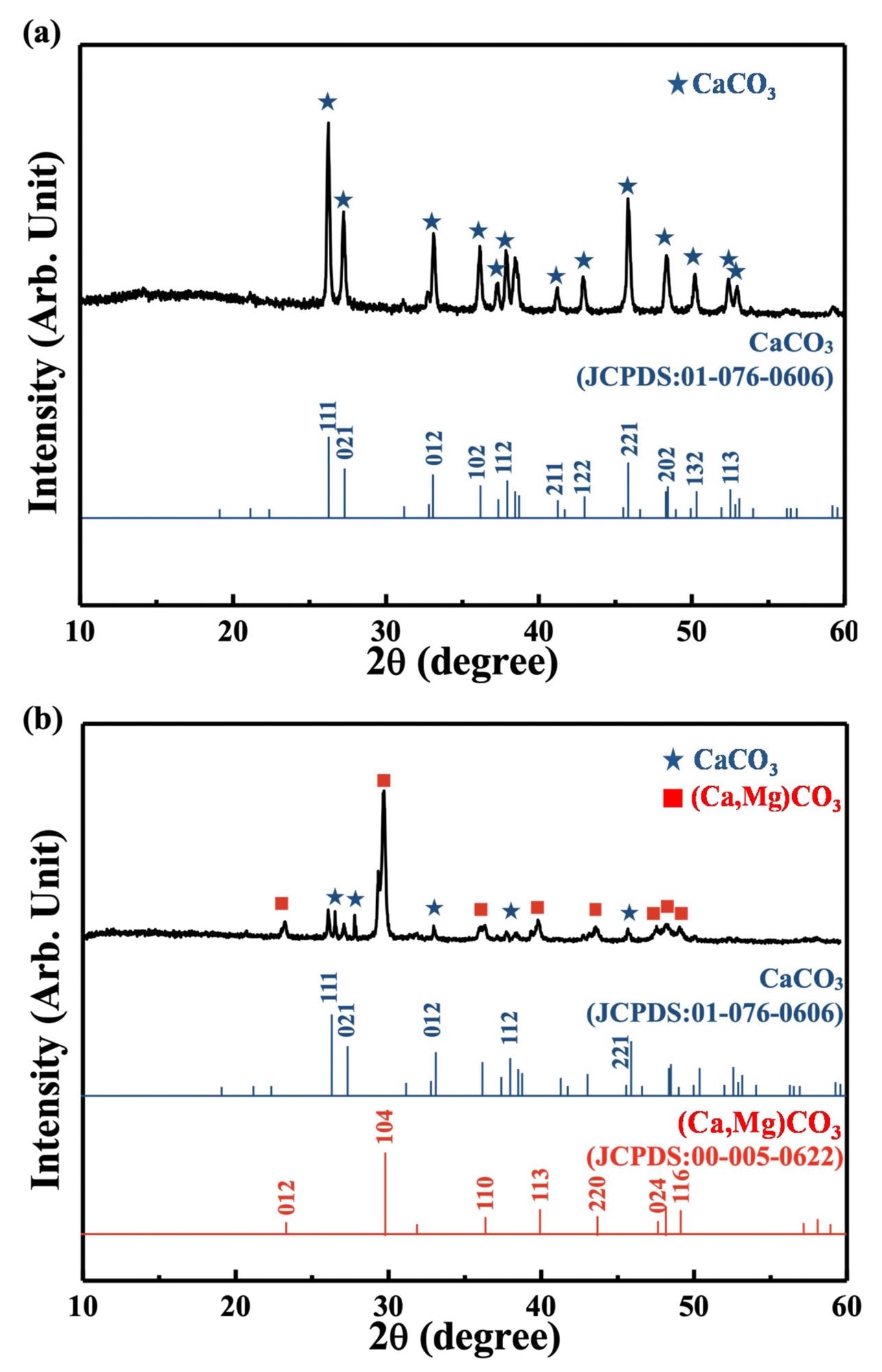
XRD pattern of (a) the as-prepared coral and (b) the commercial coral calcium sample. Image Credit: Lin, H., et al., Materials
Based on the research findings of the preceding studies, the HT-3 samples were selected for the evaluation of the heat-treatment parameters indicated in the in vitro cytotoxicity responses and in vivo bone defect tests. However, this method was time-consuming and required complex pH changes.
Additionally, standard porous-forming methods were difficult and uncommon for realizing the complex structures that were functionally relevant to human skeleton and dental enamel. The propagated Scleractinian coral was used as a calcium predecessor in this study, and the calcium carbonate microstructure had interlinked microporosity.
Result of the Research
After 8 hours of grinding, no apparent difference in the two preceding powders, Ca2P2O7 and CaCO3, were observed, although the surface area expanded, leading to supposition. The current investigation yielded similar results. Aragonite CaCO3 was a key element of the raw resources used, propagating Scleractinian coral.
After 1 hour of thermal treatment, the starting components had completely decomposed into only two crystalline forms: HA and -TCP. After 1 h of thermal treatment, the relative proportion of -TCP was larger than HAp and declined with extended thermal processing, indicating that -TCP was easily produced.
The significant influence of the initial materials and -TCP aided in the translation from CaCO3 to -TCP. Furthermore, the Ca/P proportion of the starting materials created ideal conditions for -TCP synthesis.
Because of their comparable chemical components, the diffraction patterns of CaP-based combinations overlapped. As a result, vibratory spectroscopies aided in characterizing vibrations for these substances' amorphous or crystalline forms. The FTIR and Raman spectra findings in the current investigation were in excellent accordance with the XRD results.
The occurrence of Raman OH peaks may be related to precipitated humidity due to the phosphates' extremely hygroscopic characteristics and the development of the air temperature environment after thermal processing. The OH peak dropped as the heat treatment time increased, indicating that the transition of -TCP to HA was caused by the presence of water and the availability of additional hydroxyl groups.
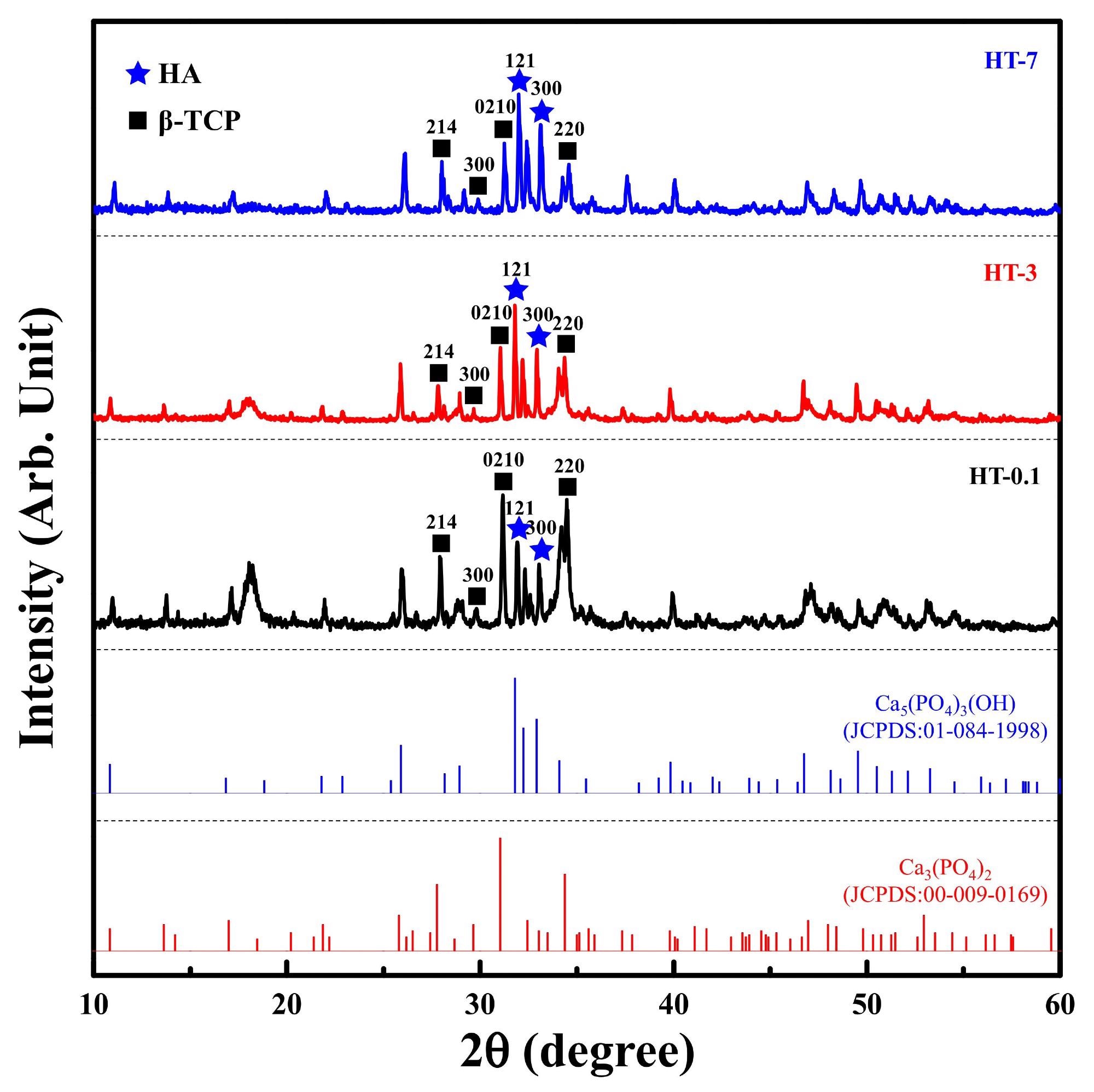
XRD patterns of the samples prepared by the heat treatment of as-prepared coral and DCPA at various durations, including reference spectra for JCPDS 01-084-1988 and 00-009-0169. Image Credit: Lin, H., et al., Materials
The Advantage of Ceramic Composites
As ceramic composite coatings, hard particles are usually placed in the near upper layers of metallic matrices. They have benefits in terms of excellent wear resistance, resistance to corrosion, and the capacity to perform at high temperatures. Ceramic matrix composites (CMCs) are also often employed in the aircraft and energy industries (turbojet engines, mechanical re-entry thermal insulation) (heat exchangers, nuclear fusion reactor walls).
In many applications, a permanently or temporarily joint between CMC parts and the surrounding materials is required.
Biomimetic ceramic composites using coral skeletons are a good breakthrough that can be used in health sectors. The biphasic CaPs produced from corals, which included 61 percent HAp and 39 percent -TCP (referred to as HT-3) were not cytotoxic.
Additionally, there were no substantial differences in local tissue reactivity between the HT-3 sample and autogenous bone. As a result of its superior biocompatibility, the synthesized coral-derived biphasic CaPs is a possibility for bone grafting.
References
Lin, H., et al. (2021). Biomimetic Ceramic Composite: Characterization, Cell Response, and In Vivo Biocompatibility. Published: 1 December 2021. Materials 2021, 14(23), 7374; https://www.mdpi.com/1996-1944/14/23/7374
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.