In a recent study published in the journal Carbon Capture Science & Technology, researchers from India developed a porous carbon adsorbent material with an excellent surface area and total pore volume. They characterized the prepared absorbent and demonstrated that it is a promising candidate for CO2 capture applications.
Study: Benzoguanamine based polyaminal carbon materials for CO2 capture application. Image Credit: Rost9/Shutterstock.com
Global Warming and Carbon Absorbents
The adverse effects of climate change and global warming are evident worldwide, and according to the Intergovernmental Panel on Climate Change (IPCC) reports, the world temperature will rise by 1.5 oC within the next 20 years.
Power generation, industries, and transport are the primary sources of greenhouse emissions due to the combustion of fossil fuels. Hence, the global society is shifting towards renewable sources and gas separation technologies to decrease carbon emissions. Carbon dioxide (CO2) capture technologies are gas separation technologies that can help control emissions of CO2, which is a major greenhouse gas.
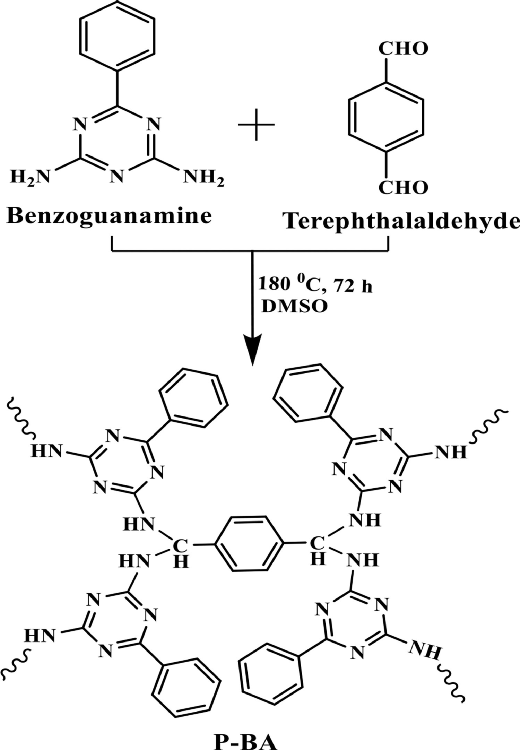
The schematic representation of P-BA preparation. Image Credit: Marimuthu S et al., Carbon Capture Science & Technology
The conventional solvent-based carbon capture technology is efficient and is based upon the principle that the amine group is more active towards CO2 and the CO2 chemically reacts with the amine group. The major limitation of this technique is the high energy requirement for solvent regeneration because it forms carbamates by chemical bonding between the amine group and CO2 molecule.
In this study, researchers prepared porous carbon materials using benzoguanamine-based polyamine polymer with good surface area and CO2 capture capacity including and excluding an activating agent potassium hydroxide (KOH) at 700 ⁰C.
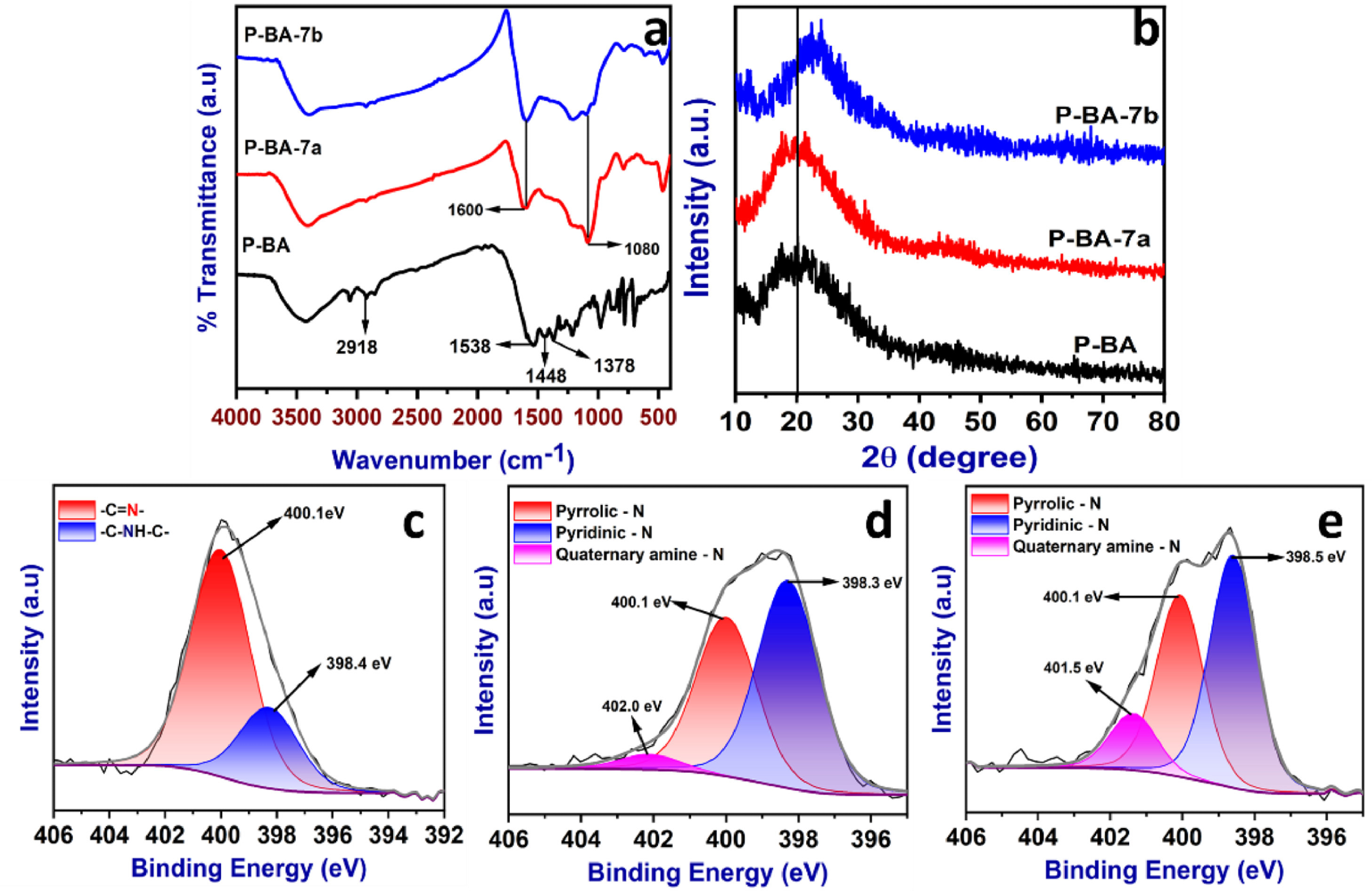
FT-IR spectra (a), powder-XRD patterns (b) and high resolution XPS spectra (N 1s) of P-BA (c), P-BA-7a (d) and P-BA-7b (e). Image Credit: Marimuthu S et al., Carbon Capture Science & Technology
The Study
Primary chemicals that were used in this work to prepare the benzoguanamine-based porous carbon adsorbent are benzoguanamine terephthaldehyde, acetone, dichloromethane (DCM), tetrahydrofuran (THF), and N, N’-dimethyl sulfoxide (DMSO), and methanol.
Using 0.5 g of benzoguanamine and a 2-mole ratio of terephthalaldehyde in 25 ml of DMSO, a compound (named P-BA by authors) was prepared. The two chemicals were heated at 80 ⁰C for 30 minutes, followed by an increase in temperature to 180 ⁰C for three days under inert conditions (N2 atmosphere). The formed solid product was filtered and washed with solvents and extraction using the Soxhlet apparatus.
Further, two carbonized samples were prepared using P-BA. The first sample was produced using a Tobler furnace in an inert (N2) atmosphere. The second sample was prepared using KOH, ethanol, and water, which were stirred for two hours, dried under vacuum, and then carbonized at 700 ⁰C for one hour at a heating rate of 3 ⁰C /min under N2 atmosphere. Post carbonization, both samples were washed with HCl (3 M) several times to remove KOH and dried at 120 ⁰C for 12 h.
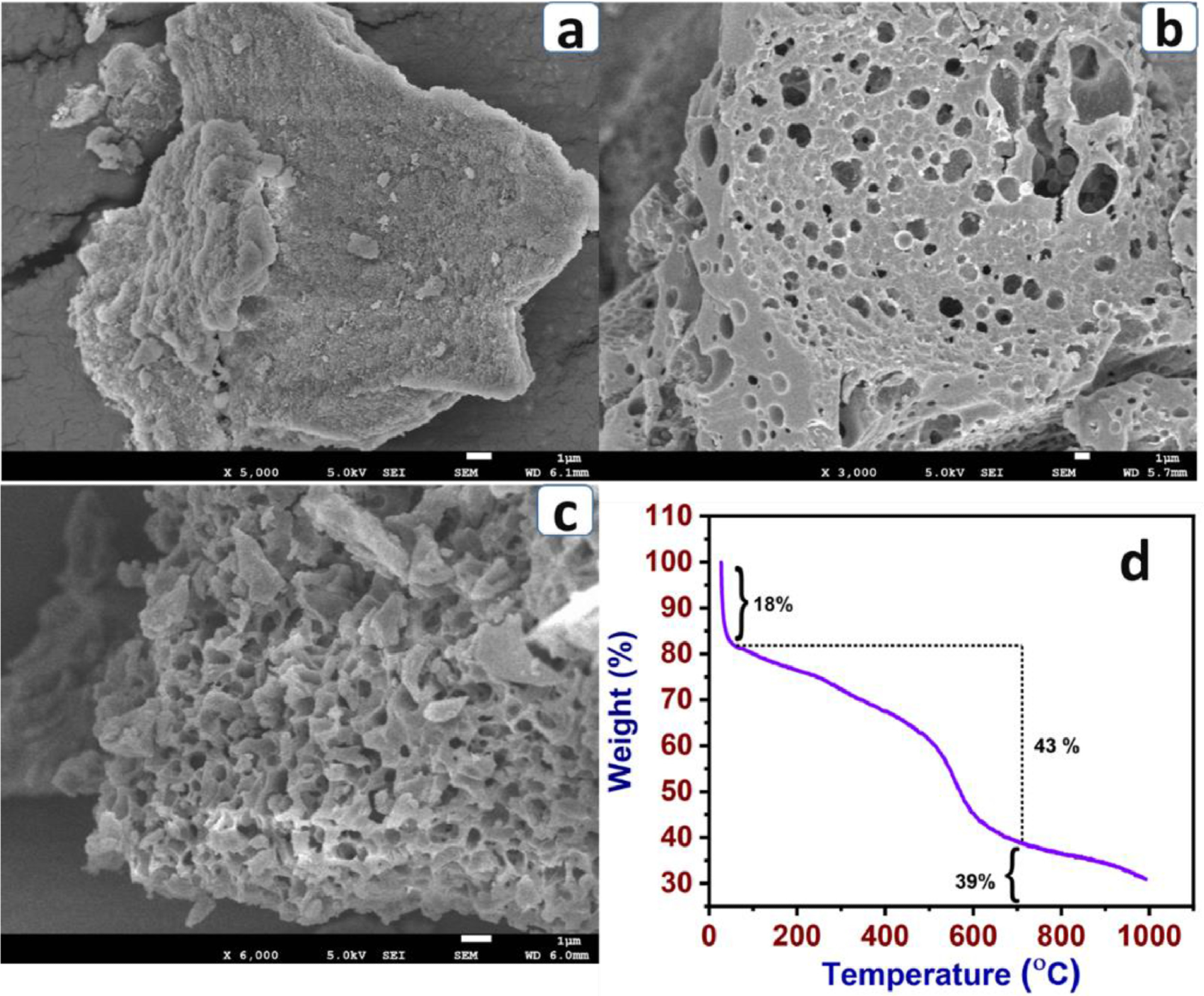
SEM images of P-BA (a), P-BA-7a (b) and P-BA-7b (c) and TGA profile of P-BA (d) (room temperature to 1000 ℃). Image Credit: Marimuthu S et al., Carbon Capture Science & Technology
Results
The FT-IR (Fourier-transform infrared spectroscopy) analysis identified the functional groups present in the polymer. The results indicated the formation of the aminal linkage between benzoguanamine and terephthaldehyde. The polymer and prepared carbonized materials were characterized using XPS (X-ray photoelectron spectroscopy) to detect carbon, nitrogen, and oxygen atoms.
The three types of nitrogen (pyridinic-N, pyrrolic-N, and quaternary amine-N) groups play a significant role in the CO2 capture process due to the lone pair of electrons identified.
Morphology studies conducted using HR-SEM (high-resolution scanning electron microscopy) analysis indicated that KOH plays a role in forming uniform pore size, which is beneficial for a higher CO2 capture capability. The physical interaction and spontaneity of adsorbent and CO2 were evaluated using Van’t Hoff and Clausius–Clapeyron equations.
The high surface area and microporous nature of prepared materials were studied by CO2, CH4, and N2 gas uptake methods at different temperatures and further analyzed using the Langmuir and Freundlich isotherm models. The results revealed the physisorption of the adsorption process and exposed the heterogeneous nature of the adsorbent.
Overall, these results confirmed the exothermic nature and physisorption of the CO2 adsorption process of benzoguanamine-based porous carbon adsorbent materials.
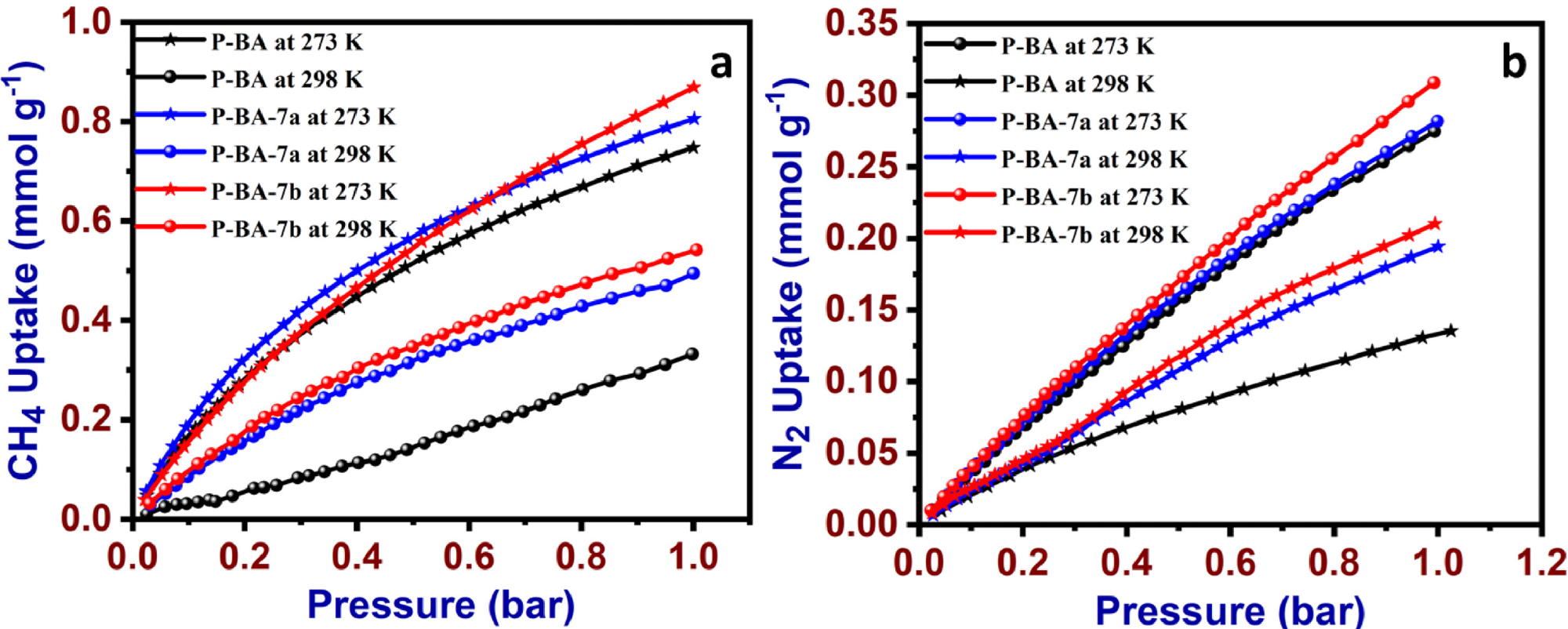
CH4 (a) N2 uptake data P-BA, P-BA-7a and P-BA-7b at 273 K and 298 K. Image Credit: Marimuthu S et al., Carbon Capture Science & Technology
Conclusions
In the study, microporous carbonized materials were successfully prepared using polyaminals. FT-IR was used to characterize the functional group of the materials. The TGA (thermogravimetric analysis results suggest the microporous carbonized materials are more stable up to 700 ⁰C (39% residual carbon).
The incremental in N2 sorption studies and HR-SEM analysis confirmed the microporous nature of carbonized materials. Both the Langmuir and Freundlich models revealed the physisorption interaction of CO2 molecules with an adsorbent and gas adsorption studies showed that the porous material prepared in the study can be good candidates for CO2 capture applications.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Marimuthu Senthilkumaran, Chokalingam Saravanan, Karuppannan Aravinth, Venkatesan Sethuraman, Pillaiyar Puthiaraj, Paulpandian Muthu Mareeswaran, Perumal Ramasamy, Benzoguanamine based polyaminal carbon materials for CO2 capture application, Carbon Capture Science & Technology (2021), DOI: https://www.sciencedirect.com/science/article/pii/S277265682100021X?via%3Dihub