A group of researchers from Taiwan analyzed two biodegradable polymeric membranes made up of poly (glycerol sebacate) (PGS) and poly(1,3-diamino-2-hydroxypropane-co-polyol sebacate) (APS) and compared their pervaporation characteristics with widely used poly dimethylpolysiloxane (PDMS) membrane, which is used for organic solvent separation from water.

Study: Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications. Image Credit: SmartPhotoLab/Shutterstock.com
Both membranes demonstrated higher separation factor, effectiveness, normalized permeation flux, and stability without deterioration up to one month. This study is available in the journal Membranes.
What is Pervaporation Polymeric Membrane Separation?
The polymeric membrane-based solvent separation method is generally categorized into two types viz. membrane distillation and membrane pervaporation. In the membrane distillation process, the micro-porous membrane is used to thermally segregate solvents at their respective vaporization temperatures, whereas in membrane pervaporation, two solvents are separated from each other by permeation and pressure difference-dependent partial vaporization, which are driven by the selective affinity of a finely porous or nonporous dense membrane towards a solvent and its concentration gradient through it.
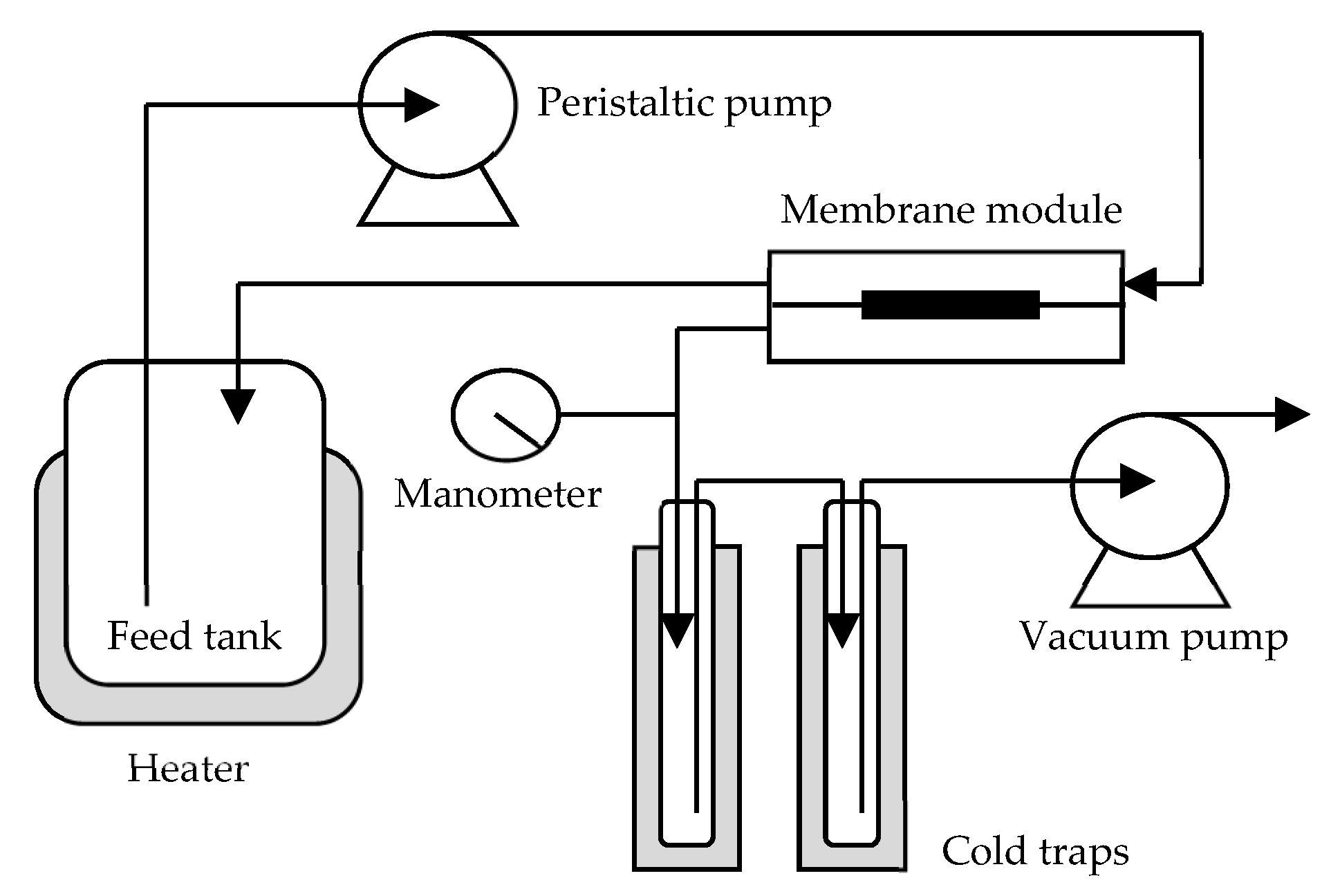
Schematic diagram of the pervaporation process in this study. Image Credit: Chang, P et al., Membranes
The membrane pervaporation process does not require thermal energy; thus, it is energy efficient. Also, based on the separation type i.e. the separation of organic solvent from water or water from organic solvent, hydrophobic or hydrophilic polymers are used, respectively.
PDMS is a widely used hydrophobic, nonbiodegradable, biocompatible, and highly flexible polymer membrane, whereas polyvinyl alcohol (PVA), polyimide (PI), and polyacrylonitrile (PAN) are hydrophilic polymer membranes.
Although most polymeric pervaporation membranes have long-term durability and could be reused multiple times, the spent nonbiodegradable membranes eventually turn into secondary pollutants. Moreover, the burning of these solid membrane wastes releases a large number of gas contaminants; thus biodegradable polymers are the preferred candidate for these membranes.
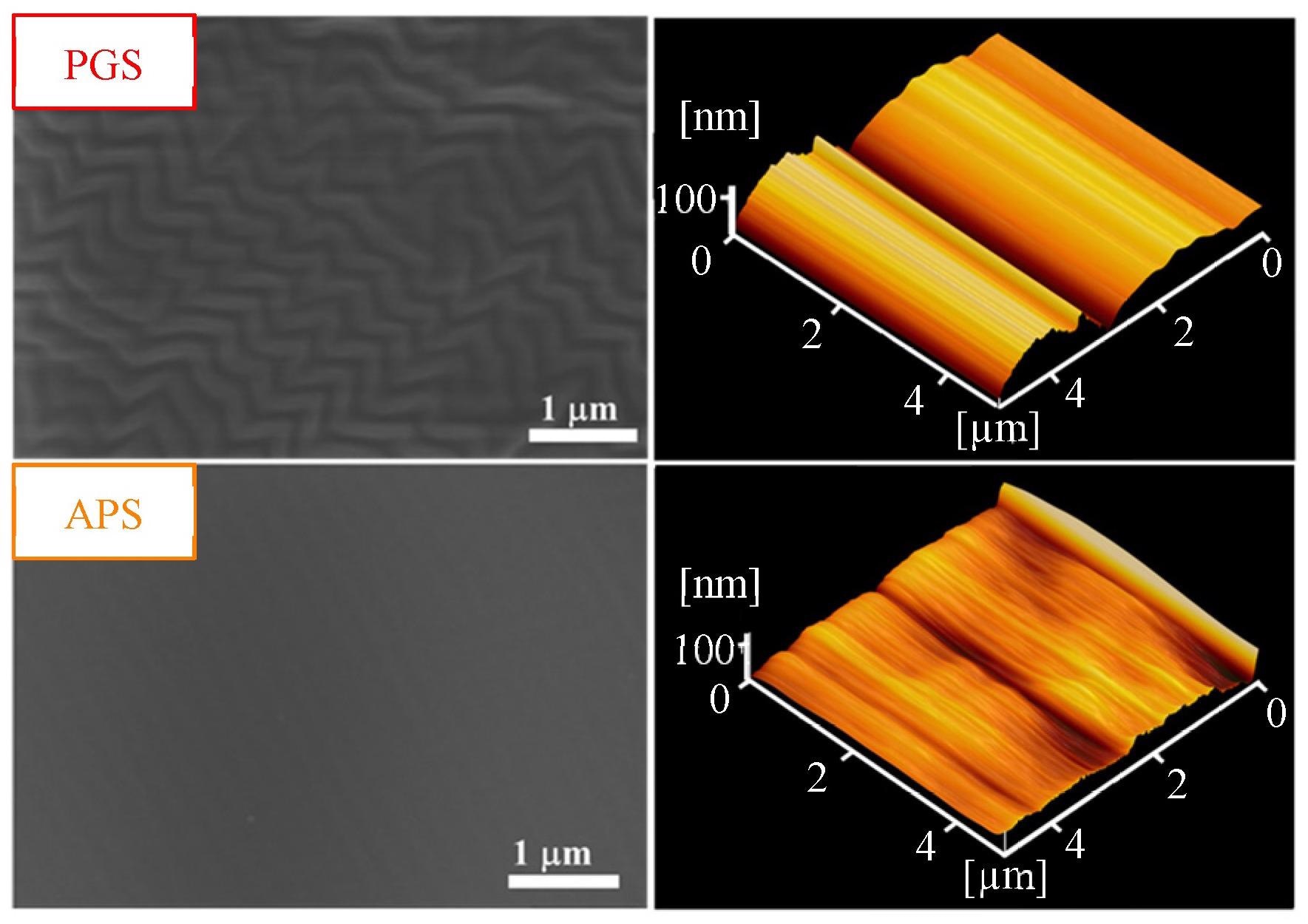
FE-SEM (×20S000) and AFM images of the two biodegradable membranes prepared in this study. Image Credit: Chang, P et al., Membranes
About the Study
In this study, researchers first synthesized the dense membranes of PDMS, PGS, and APS followed by the analysis of membrane thickness, surface morphology, the detection of functional groups, thermal properties, glass transition temperature, and water contact angle. PDMS membrane was synthesized by solid-state mixing of PDMS precursors with the curing agent in the ratio of 10:1 followed by ultrasonication and heating at 70℃ for hours on a PET substrate for curing.
Subsequently, the total permeation flux and separation factor were evaluated through the pervaporation experiment. Also, the degree of swelling and swelling ratio for all membranes was measured at 37 ℃ for 8 hours in ethanol, isopropanol, n-butanol, acetone, acetic acid, and water solvents, respectively. Finally, the solvent recovery by pervaporation was performed under the acetone–butanol–ethanol (ABE) fermentation system.
Observations
Field-emission scanning electron microscopy (FE-SEM) and atomic force microscope (AFM) revealed that all the prepared membranes of PDMS, PGS, and APS had a thickness of 200 µm ± 3%. Also, PGS had the highest surface roughness, whereas PDMS had the lowest surface roughness.
The attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR) figures indicated the presence of characteristic CH and C=O bond peaks for PGS and APS at 2960 cm-1 and 1740 cm-1, respectively, whereas the APS membrane displayed an additional peak for N-H bond at 1650 cm-1.
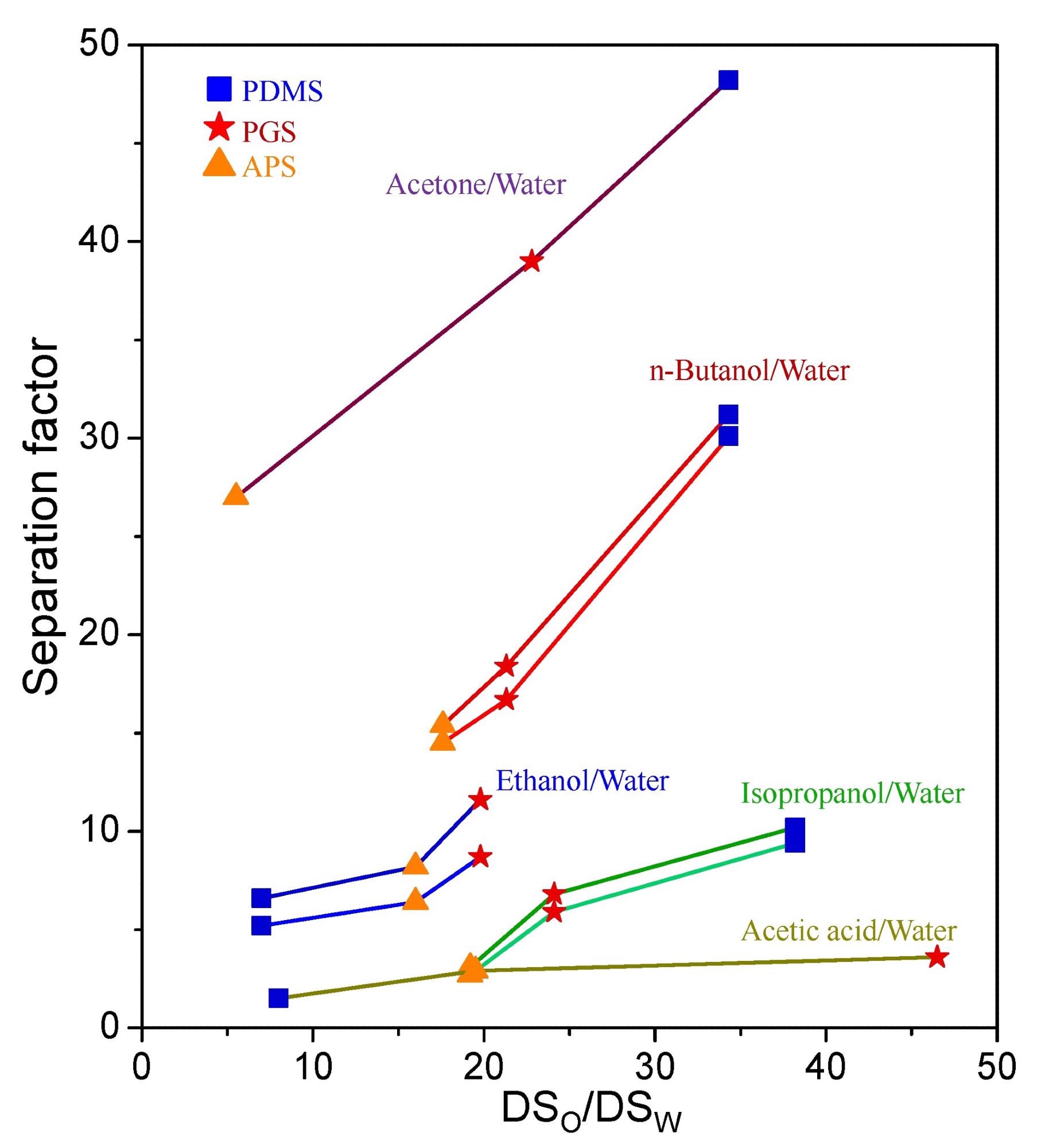
The relationship between the separation factor and the swelling ratio of organic solvent to water (DSo/DSw). Image Credit: Chang, P et al., Membranes
Thermogravimetric analyzer (TGA) analysis showed similar thermal stability for PGS and APS. Both polymers showed a minor weight loss between 110-300 ℃, a large weight loss between 400-450 ℃, and a complete burn down at 600 ℃.
Furthermore, differential scanning calorimeter (DSC) analysis revealed the glass transition temperature (Tg) for PDMS, PGS, and APS at -125 ℃, -20 ℃, and 8 ℃, respectively; thus at 37 ℃ the operating temperature of all of them were above their respective Tg values and in a rubbery state. The polymer chain flexibility at the rubbery state facilitated the smooth passage of the vaporized solvent molecules.
Moreover, the water contact angle of APS was the lowest at 85⁰ followed by PGS at 94⁰ and PDMS at 104⁰, respectively. The higher the water contact angle the higher the hydrophobicity; thus PDMS was more hydrophobic than PGS and APS, respectively.
Additionally, APS had the highest degree of swelling for ethanol, isopropanol, n-butanol, acetic acid, and water, whereas PGS had the highest degree of swelling for acetone. PDMS showed much lower swelling than that of both PGS and APS for all solvents.
Conclusions
The researchers prepared dense polymer membranes of PGS and APS and compared their pervaporation characteristics with widely used PDMS membranes. All three membranes were competing with each other in the category of hydrophobic membranes, which are used for the separation of organic solvents from water.
Also, both polymers were biodegradable in contrast to PDMS and they demonstrated stability retention up to one month in an ABE system, thus they are viable eco-friendly alternatives to PDMS in practical pervaporation applications.
Reference
Chang, P., Wang, J., Li, S., Suen, S., Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications. Membranes, 2021, 11, 970. https://www.mdpi.com/2077-0375/11/12/970/htm
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.