.jpg) By Susha Cheriyedath, M.Sc.Reviewed by Skyla BailyFeb 2 2022
By Susha Cheriyedath, M.Sc.Reviewed by Skyla BailyFeb 2 2022In an article recently published in the open-access journal Materials, researchers investigated the potential of an industrial waste, waste tires, towards the production of a viable adsorbent material that can be useful in the treatment of wastewater through the removal of organic pollutants.

Study: Potential Valorization of Waste Tires as Activated Carbon-Based Adsorbent for Organic Contaminants Removal. Image Credit: sandsun/Shutterstock.com
The resaerchers also demonstrated the generation of activated chars from waste tires via pyrolysis and activation techniques.
Background
The rapid development in the automobile sector over the last two decades has resulted in a massive number of waste tires. Tires have a diverse composition, making their material and/or energy recovery a lucrative business. Moreover, tires are a promising non-fossil energy source due to their high calorific value (28-37 MJ kg-11) and low mineral content.
Thermal recycling of waste tire materials using pyrolysis might be considered the most convenient approach to mitigate the negative consequences of waste management on the environment and to produce fuel and other solid products. Chars obtained from waste tire pyrolysis could be utilized as adsorbents for applications in gas storage or pollution management.

Picture of the ground rubber feedstock. Image Credit: Frikha, K et al., Materials
The activation method, which combines heat and an activating substance, is frequently employed to improve the textural and chemical properties of carbon compounds. Waste tire-derived activated carbon (AC) has been investigated as adsorbents in both aqueous and gaseous conditions. However, sparse reports are available in the literature assessing the environmental impact of preparing AC from ground rubber feedstock from the perspective of a life cycle assessment. The production of AC from waste tires not only promotes the preparation of materials for water treatment but also assists in the management of waste tires.
About the Study
In the present study, researchers investigated the pyrolysis of ground rubber tires in the first stage utilizing non-isothermal and isothermal thermogravimetric analysis techniques. Ground rubber with a mean particle size of 1.5–4 mm was employed as the char precursor feedstock. The effect of operating factors on pyrolysis product yields, such as the heating rate and pyrolysis temperature, was studied. For improved char recovery, a largescale fixed-bed reactor was used to carry out the slow pyrolysis of ground rubber tires. Four pyrolysis temperatures were chosen based on the thermogravimetric data.
To determine the correlations between pyrolysis temperature and char qualities, the generated chars were examined in terms of the mineral contents, textural features, structural properties, and proximate analysis.
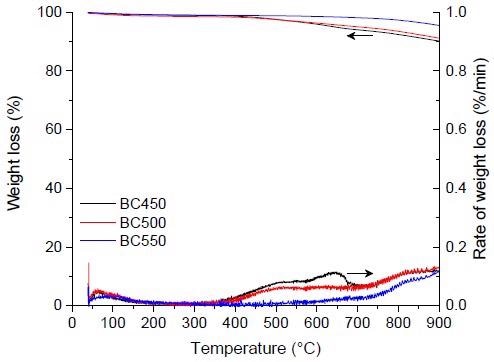
TG and DTG curves for the pyrolysis of the ground rubber-based chars. Image Credit: Frikha, K et al., Materials
Subsequently, a sequence of activated chars was synthesized in the second step, commencing with pyrolytic chars using chemical and/or physical activation procedures. The impact of these treatments on the chemical, textural, and physical characteristics of the generated carbon materials (char and activated chars) was elucidated. A small-scale batch reactor setup was used to evaluate the adsorption properties of the activated chars in aqueous media, using atrazine and ibuprofen as sample adsorbates.
Observations
In this study, activated carbon was successfully generated from the waste tires by pyrolysis and activation methods and used as organic micropollutant adsorbents for atrazine and ibuprofen. The thermogravimetric examination of a ground rubber tire revealed that the pyrolysis temperature and heating rate had no qualitative effect on the thermal deterioration of the ground rubber sample.
However, they were found to have a significant impact on the product (char) yields; higher temperatures encouraged the synthesis of more gases and liquids, whereas lower temperatures preferred the formation of more char (solid fraction). The pyrolysis of pulverized rubber samples at various heating rates was mostly complete by 550 °C, with the highest char output obtained at 450 °C at a 5 °C min-1 heating rate.
Chemical activation was observed to be an effective way to minimize the inorganic compounds found in the ground rubber tires while also contributing to a significant increase in the porosity and surface area of the chars. Chemically activated chars were found to have a high aqueous adsorption capacity of 208 mg g−1 for atrazine.
The physicochemical characteristics of the tire-derived char samples did not appear to be particularly sensitive to the pyrolysis temperature, according to the char characterization studies. Since the pyrolytic chars had significant ash content and limited surface areas, activation treatments were required to increase their adsorbent capabilities. Activated char analysis revealed that the activation procedure resulted in an increase in the pore volume, surface area, and surface alkalinity, as well as a reduction in the zinc and sulfur contents.
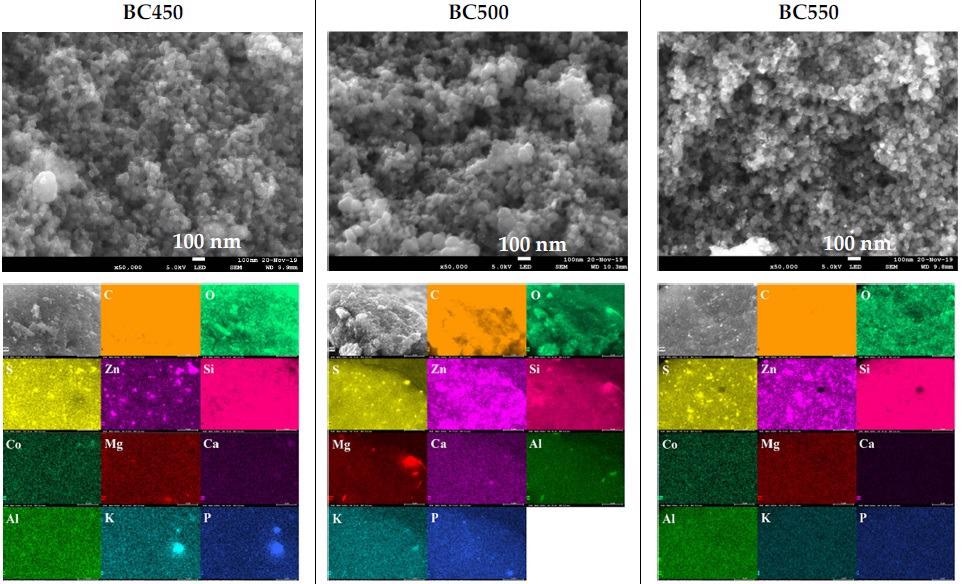
SEM images and elemental distribution maps of the ground rubber-based chars. Image Credit: Frikha, K et al., Materials
Conclusions
In conclusion, this study examined the effect of the activation procedure on the porosity, structure, and surface structural properties of the resulting activated chars. The activated chars had a higher adsorption capacity than those described in the literature, according to the experimental results obtained for atrazine adsorption.
However, the adsorption capability of ibuprofen on activated chars was found to be lower than the previously reported values. Among the various activation methods studied in this article, chemical activation generated char with a significantly developed micro/meso-porosity and increased surface area, resulting in superior adsorption capabilities.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Frikha, K., Limousy, L., Claret, J. P. et al. Potential Valorization of Waste Tires as Activated Carbon-Based Adsorbent for Organic Contaminants Removal. Materials 15(3), 1099 (2022). https://www.mdpi.com/1996-1944/15/3/1099